Fluorine »
PDB 1h2j-1j97 »
1hwk »
Fluorine in PDB 1hwk: Complex of the Catalytic Portion of Human Hmg-Coa Reductase with Atorvastatin
Enzymatic activity of Complex of the Catalytic Portion of Human Hmg-Coa Reductase with Atorvastatin
All present enzymatic activity of Complex of the Catalytic Portion of Human Hmg-Coa Reductase with Atorvastatin:
1.1.1.34;
1.1.1.34;
Protein crystallography data
The structure of Complex of the Catalytic Portion of Human Hmg-Coa Reductase with Atorvastatin, PDB code: 1hwk
was solved by
E.S.Istvan,
J.Deisenhofer,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 43.40 / 2.22 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 74.596, 172.724, 80.008, 90.00, 117.73, 90.00 |
| R / Rfree (%) | 21.2 / 23.5 |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Complex of the Catalytic Portion of Human Hmg-Coa Reductase with Atorvastatin
(pdb code 1hwk). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 4 binding sites of Fluorine where determined in the Complex of the Catalytic Portion of Human Hmg-Coa Reductase with Atorvastatin, PDB code: 1hwk:
Jump to Fluorine binding site number: 1; 2; 3; 4;
In total 4 binding sites of Fluorine where determined in the Complex of the Catalytic Portion of Human Hmg-Coa Reductase with Atorvastatin, PDB code: 1hwk:
Jump to Fluorine binding site number: 1; 2; 3; 4;
Fluorine binding site 1 out of 4 in 1hwk
Go back to
Fluorine binding site 1 out
of 4 in the Complex of the Catalytic Portion of Human Hmg-Coa Reductase with Atorvastatin
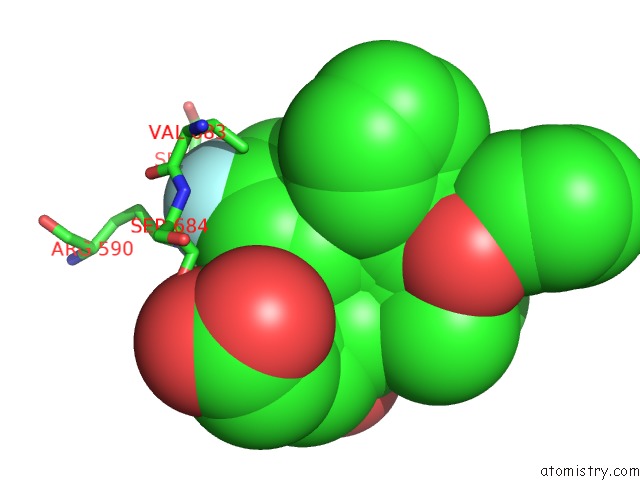
Mono view
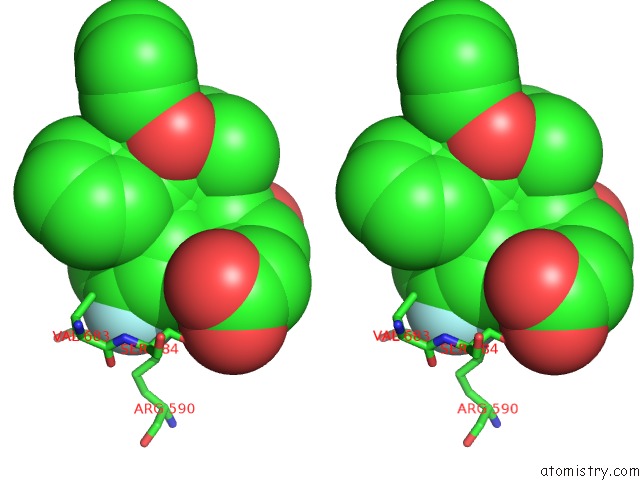
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Complex of the Catalytic Portion of Human Hmg-Coa Reductase with Atorvastatin within 5.0Å range:
|
Fluorine binding site 2 out of 4 in 1hwk
Go back to
Fluorine binding site 2 out
of 4 in the Complex of the Catalytic Portion of Human Hmg-Coa Reductase with Atorvastatin
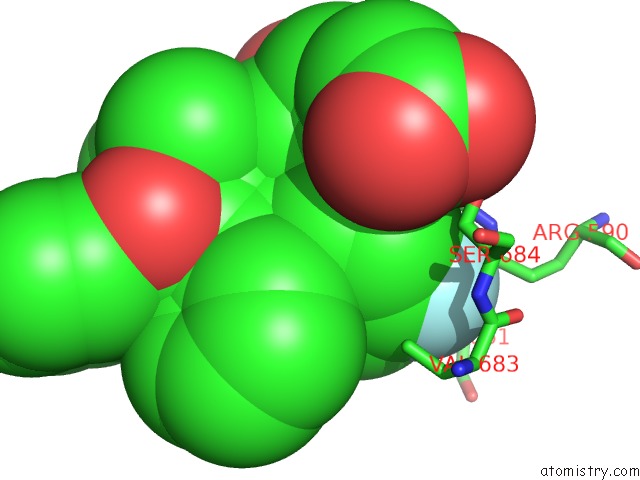
Mono view
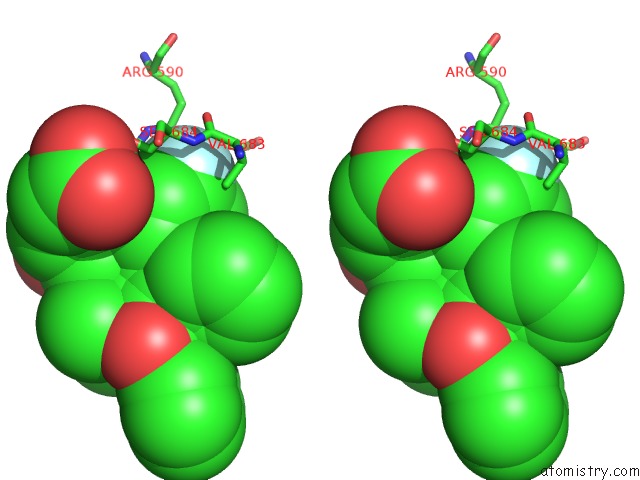
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Complex of the Catalytic Portion of Human Hmg-Coa Reductase with Atorvastatin within 5.0Å range:
|
Fluorine binding site 3 out of 4 in 1hwk
Go back to
Fluorine binding site 3 out
of 4 in the Complex of the Catalytic Portion of Human Hmg-Coa Reductase with Atorvastatin
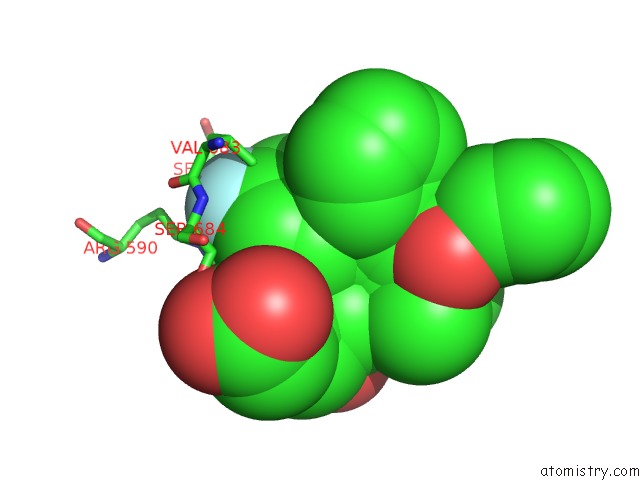
Mono view
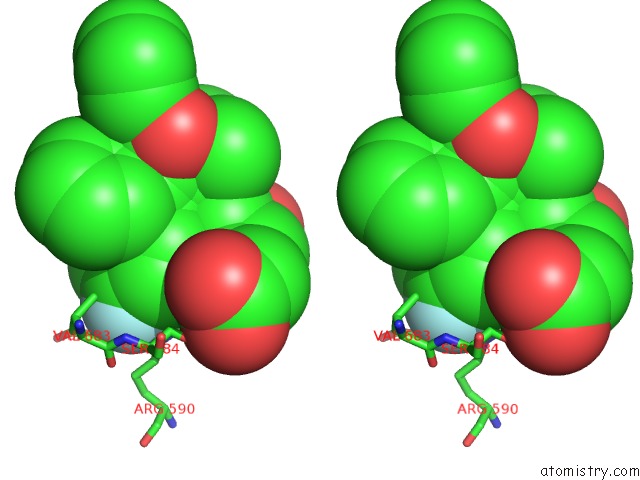
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Complex of the Catalytic Portion of Human Hmg-Coa Reductase with Atorvastatin within 5.0Å range:
|
Fluorine binding site 4 out of 4 in 1hwk
Go back to
Fluorine binding site 4 out
of 4 in the Complex of the Catalytic Portion of Human Hmg-Coa Reductase with Atorvastatin
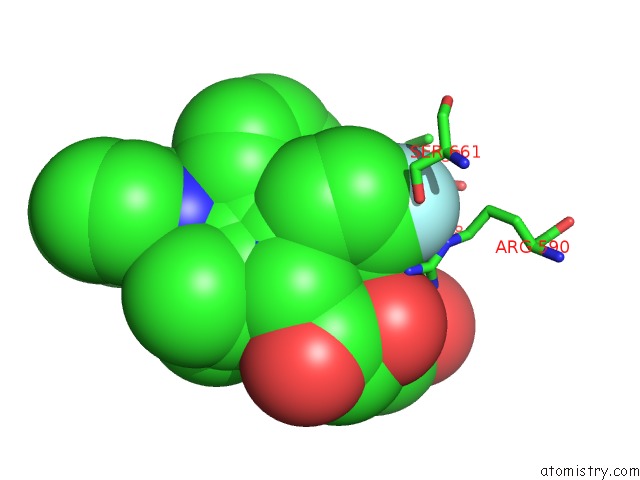
Mono view
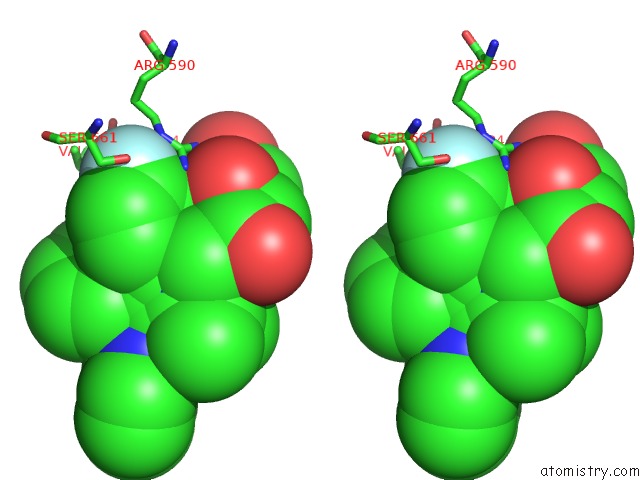
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 4 of Complex of the Catalytic Portion of Human Hmg-Coa Reductase with Atorvastatin within 5.0Å range:
|
Reference:
E.S.Istvan,
J.Deisenhofer.
Structural Mechanism For Statin Inhibition of Hmg-Coa Reductase. Science V. 292 1160 2001.
ISSN: ISSN 0036-8075
PubMed: 11349148
DOI: 10.1126/SCIENCE.1059344
Page generated: Mon Jul 14 10:55:12 2025
ISSN: ISSN 0036-8075
PubMed: 11349148
DOI: 10.1126/SCIENCE.1059344
Last articles
Fe in 6ZKLFe in 6ZKI
Fe in 6ZKK
Fe in 6ZKJ
Fe in 6ZKH
Fe in 6ZKG
Fe in 6ZKF
Fe in 6ZKE
Fe in 6ZKD
Fe in 6ZKC