Fluorine »
PDB 2duz-2fq6 »
2evc »
Fluorine in PDB 2evc: Crystal Structure of E. Coli. Methionine Amino Peptidase in Complex with 5-(2-(Trifluoromethyl)Phenyl)Furan-2- Carboxylic Acid
Enzymatic activity of Crystal Structure of E. Coli. Methionine Amino Peptidase in Complex with 5-(2-(Trifluoromethyl)Phenyl)Furan-2- Carboxylic Acid
All present enzymatic activity of Crystal Structure of E. Coli. Methionine Amino Peptidase in Complex with 5-(2-(Trifluoromethyl)Phenyl)Furan-2- Carboxylic Acid:
3.4.11.18;
3.4.11.18;
Protein crystallography data
The structure of Crystal Structure of E. Coli. Methionine Amino Peptidase in Complex with 5-(2-(Trifluoromethyl)Phenyl)Furan-2- Carboxylic Acid, PDB code: 2evc
was solved by
W.-J.Huang,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 20.00 / 1.60 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 37.800, 60.200, 50.400, 90.00, 104.50, 90.00 |
| R / Rfree (%) | 21 / 22.9 |
Other elements in 2evc:
The structure of Crystal Structure of E. Coli. Methionine Amino Peptidase in Complex with 5-(2-(Trifluoromethyl)Phenyl)Furan-2- Carboxylic Acid also contains other interesting chemical elements:
| Manganese | (Mn) | 2 atoms |
| Sodium | (Na) | 1 atom |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Crystal Structure of E. Coli. Methionine Amino Peptidase in Complex with 5-(2-(Trifluoromethyl)Phenyl)Furan-2- Carboxylic Acid
(pdb code 2evc). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 3 binding sites of Fluorine where determined in the Crystal Structure of E. Coli. Methionine Amino Peptidase in Complex with 5-(2-(Trifluoromethyl)Phenyl)Furan-2- Carboxylic Acid, PDB code: 2evc:
Jump to Fluorine binding site number: 1; 2; 3;
In total 3 binding sites of Fluorine where determined in the Crystal Structure of E. Coli. Methionine Amino Peptidase in Complex with 5-(2-(Trifluoromethyl)Phenyl)Furan-2- Carboxylic Acid, PDB code: 2evc:
Jump to Fluorine binding site number: 1; 2; 3;
Fluorine binding site 1 out of 3 in 2evc
Go back to
Fluorine binding site 1 out
of 3 in the Crystal Structure of E. Coli. Methionine Amino Peptidase in Complex with 5-(2-(Trifluoromethyl)Phenyl)Furan-2- Carboxylic Acid

Mono view
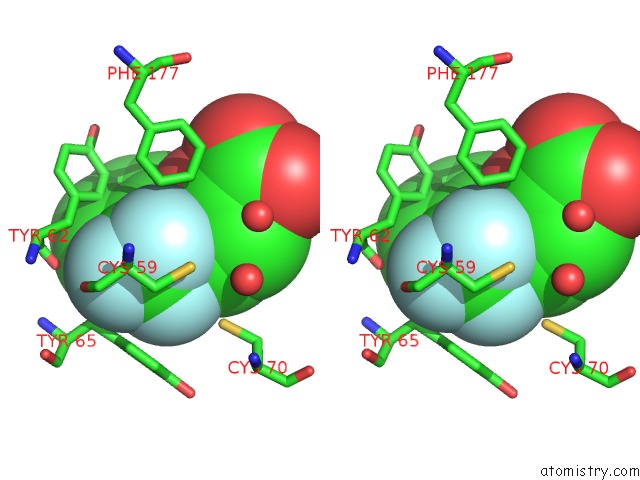
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Crystal Structure of E. Coli. Methionine Amino Peptidase in Complex with 5-(2-(Trifluoromethyl)Phenyl)Furan-2- Carboxylic Acid within 5.0Å range:
|
Fluorine binding site 2 out of 3 in 2evc
Go back to
Fluorine binding site 2 out
of 3 in the Crystal Structure of E. Coli. Methionine Amino Peptidase in Complex with 5-(2-(Trifluoromethyl)Phenyl)Furan-2- Carboxylic Acid
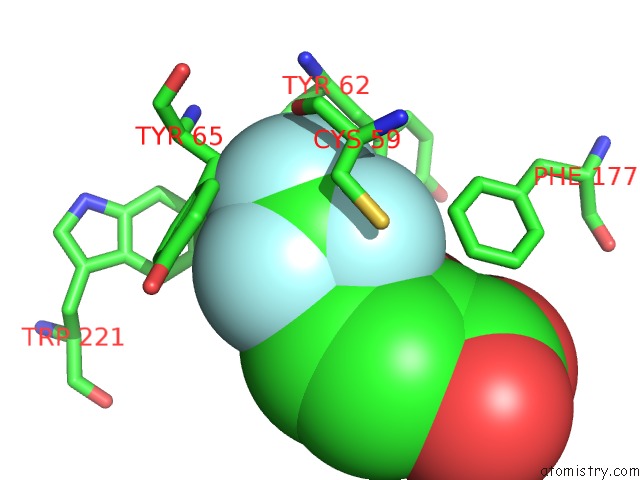
Mono view
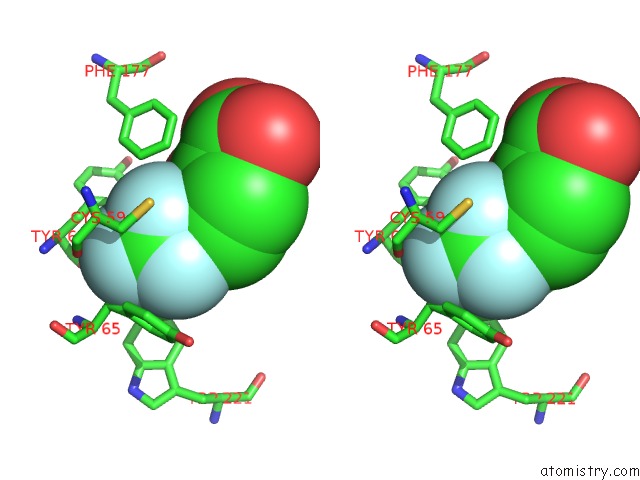
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Crystal Structure of E. Coli. Methionine Amino Peptidase in Complex with 5-(2-(Trifluoromethyl)Phenyl)Furan-2- Carboxylic Acid within 5.0Å range:
|
Fluorine binding site 3 out of 3 in 2evc
Go back to
Fluorine binding site 3 out
of 3 in the Crystal Structure of E. Coli. Methionine Amino Peptidase in Complex with 5-(2-(Trifluoromethyl)Phenyl)Furan-2- Carboxylic Acid
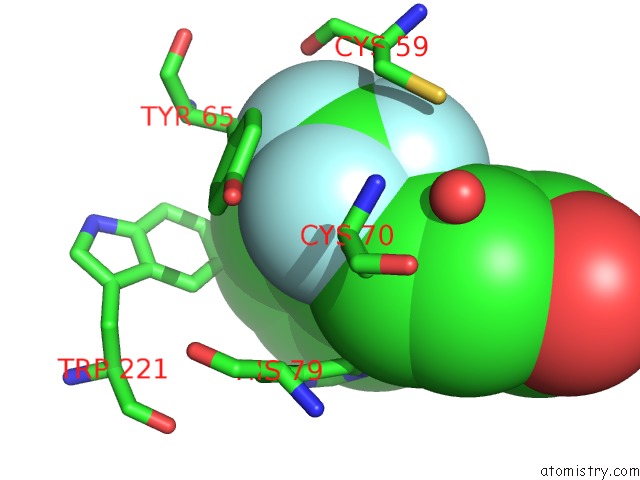
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Crystal Structure of E. Coli. Methionine Amino Peptidase in Complex with 5-(2-(Trifluoromethyl)Phenyl)Furan-2- Carboxylic Acid within 5.0Å range:
|
Reference:
S.X.Xie,
W.J.Huang,
Z.Q.Ma,
M.Huang,
R.P.Hanzlik,
Q.Z.Ye.
Structural Analysis of Metalloform-Selective Inhibition of Methionine Aminopeptidase. Acta Crystallogr.,Sect.D V. 62 425 2006.
ISSN: ISSN 0907-4449
PubMed: 16552144
DOI: 10.1107/S0907444906003878
Page generated: Mon Jul 14 12:53:37 2025
ISSN: ISSN 0907-4449
PubMed: 16552144
DOI: 10.1107/S0907444906003878
Last articles
I in 3QD5I in 3QF1
I in 3QH9
I in 3QGN
I in 3Q7S
I in 3Q5M
I in 3Q1E
I in 3PMC
I in 3PM6
I in 3PY4