Fluorine »
PDB 2q9p-2rbe »
2qu5 »
Fluorine in PDB 2qu5: Crystal Structure of the VEGFR2 Kinase Domain in Complex with A Benzimidazole Inhibitor
Enzymatic activity of Crystal Structure of the VEGFR2 Kinase Domain in Complex with A Benzimidazole Inhibitor
All present enzymatic activity of Crystal Structure of the VEGFR2 Kinase Domain in Complex with A Benzimidazole Inhibitor:
2.7.10.1;
2.7.10.1;
Protein crystallography data
The structure of Crystal Structure of the VEGFR2 Kinase Domain in Complex with A Benzimidazole Inhibitor, PDB code: 2qu5
was solved by
D.A.Whittington,
J.L.Kim,
A.M.Long,
P.Rose,
Y.Gu,
H.Zhao,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 30.00 / 2.95 |
| Space group | P 21 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 66.852, 143.828, 58.483, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 21.3 / 25.1 |
Other elements in 2qu5:
The structure of Crystal Structure of the VEGFR2 Kinase Domain in Complex with A Benzimidazole Inhibitor also contains other interesting chemical elements:
| Chlorine | (Cl) | 1 atom |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Crystal Structure of the VEGFR2 Kinase Domain in Complex with A Benzimidazole Inhibitor
(pdb code 2qu5). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 3 binding sites of Fluorine where determined in the Crystal Structure of the VEGFR2 Kinase Domain in Complex with A Benzimidazole Inhibitor, PDB code: 2qu5:
Jump to Fluorine binding site number: 1; 2; 3;
In total 3 binding sites of Fluorine where determined in the Crystal Structure of the VEGFR2 Kinase Domain in Complex with A Benzimidazole Inhibitor, PDB code: 2qu5:
Jump to Fluorine binding site number: 1; 2; 3;
Fluorine binding site 1 out of 3 in 2qu5
Go back to
Fluorine binding site 1 out
of 3 in the Crystal Structure of the VEGFR2 Kinase Domain in Complex with A Benzimidazole Inhibitor
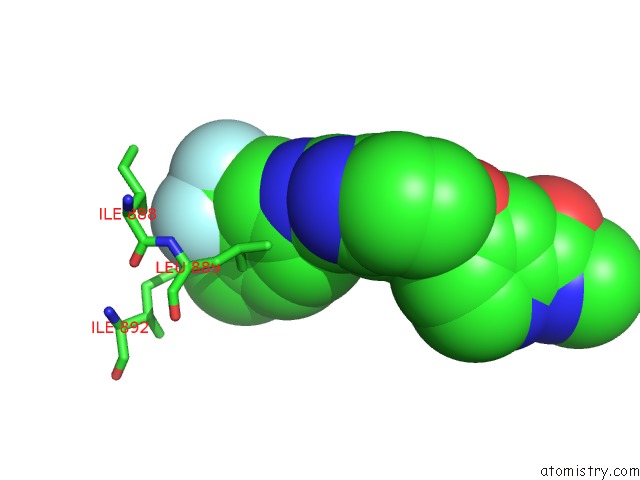
Mono view
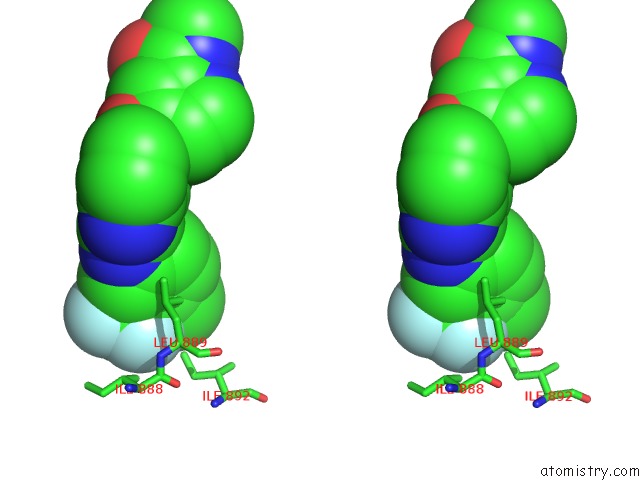
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Crystal Structure of the VEGFR2 Kinase Domain in Complex with A Benzimidazole Inhibitor within 5.0Å range:
|
Fluorine binding site 2 out of 3 in 2qu5
Go back to
Fluorine binding site 2 out
of 3 in the Crystal Structure of the VEGFR2 Kinase Domain in Complex with A Benzimidazole Inhibitor
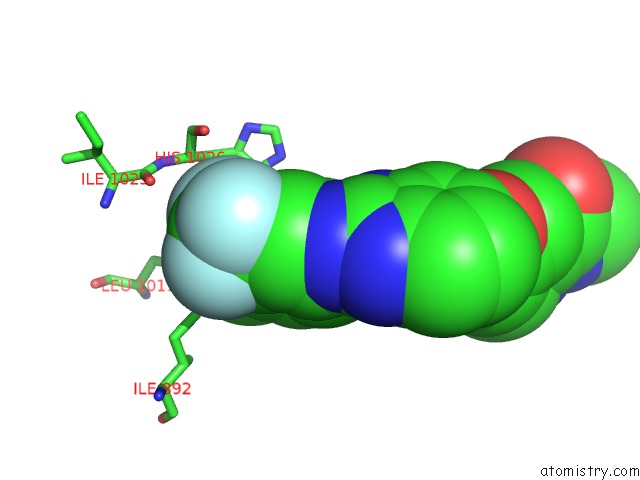
Mono view
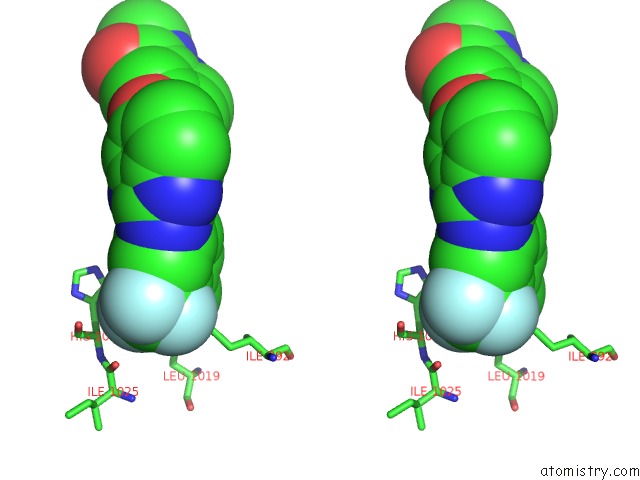
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Crystal Structure of the VEGFR2 Kinase Domain in Complex with A Benzimidazole Inhibitor within 5.0Å range:
|
Fluorine binding site 3 out of 3 in 2qu5
Go back to
Fluorine binding site 3 out
of 3 in the Crystal Structure of the VEGFR2 Kinase Domain in Complex with A Benzimidazole Inhibitor
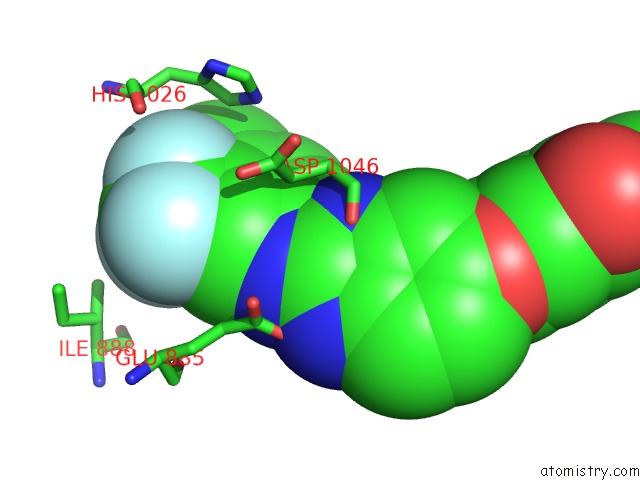
Mono view
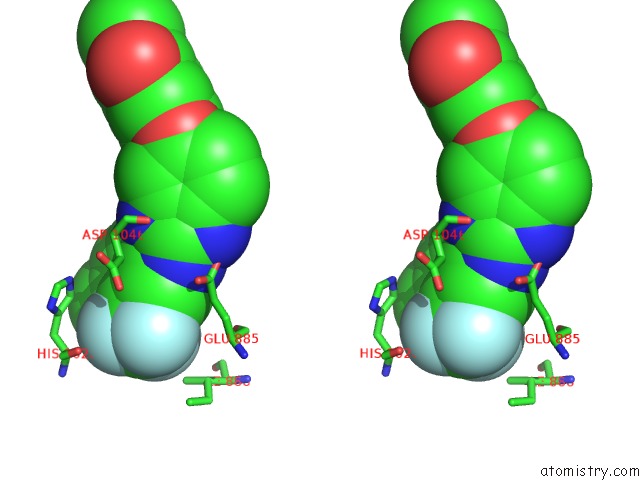
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Crystal Structure of the VEGFR2 Kinase Domain in Complex with A Benzimidazole Inhibitor within 5.0Å range:
|
Reference:
M.H.Potashman,
J.Bready,
A.Coxon,
T.M.Demelfi,
L.Dipietro,
N.Doerr,
D.Elbaum,
J.Estrada,
P.Gallant,
J.Germain,
Y.Gu,
J.C.Harmange,
S.A.Kaufman,
R.Kendall,
J.L.Kim,
G.N.Kumar,
A.M.Long,
S.Neervannan,
V.F.Patel,
A.Polverino,
P.Rose,
S.V.Plas,
D.Whittington,
R.Zanon,
H.Zhao.
Design, Synthesis, and Evaluation of Orally Active Benzimidazoles and Benzoxazoles As Vascular Endothelial Growth Factor-2 Receptor Tyrosine Kinase Inhibitors. J.Med.Chem. V. 50 4351 2007.
ISSN: ISSN 0022-2623
PubMed: 17696416
DOI: 10.1021/JM070034I
Page generated: Mon Jul 14 14:15:38 2025
ISSN: ISSN 0022-2623
PubMed: 17696416
DOI: 10.1021/JM070034I
Last articles
K in 9HKXK in 9HKY
K in 9HKW
K in 9HFO
K in 9HFN
K in 9HI3
K in 9HFM
K in 9HAC
K in 9GXH
K in 9HAG