Fluorine »
PDB 3l8v-3lxp »
3lu7 »
Fluorine in PDB 3lu7: Human Serum Albumin in Complex with Compound 2
Protein crystallography data
The structure of Human Serum Albumin in Complex with Compound 2, PDB code: 3lu7
was solved by
D.Buttar,
N.Colclough,
S.Gerhardt,
P.A.Macfaul,
S.D.Phillips,
A.Plowright,
P.Whittamore,
K.Tam,
K.Maskos,
S.Steinbacher,
H.Steuber,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 55.44 / 2.80 |
| Space group | P 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 58.541, 59.185, 95.603, 75.30, 87.86, 75.51 |
| R / Rfree (%) | 22.5 / 27.6 |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Human Serum Albumin in Complex with Compound 2
(pdb code 3lu7). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 2 binding sites of Fluorine where determined in the Human Serum Albumin in Complex with Compound 2, PDB code: 3lu7:
Jump to Fluorine binding site number: 1; 2;
In total 2 binding sites of Fluorine where determined in the Human Serum Albumin in Complex with Compound 2, PDB code: 3lu7:
Jump to Fluorine binding site number: 1; 2;
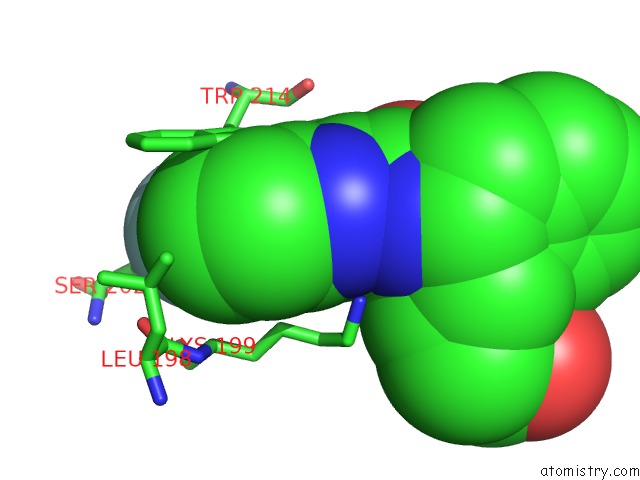
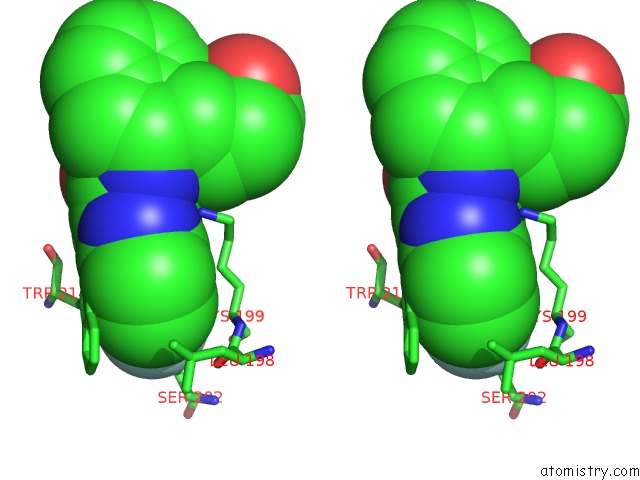
Fluorine binding site 1 out of 2 in 3lu7
Go back to
Fluorine binding site 1 out
of 2 in the Human Serum Albumin in Complex with Compound 2
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Human Serum Albumin in Complex with Compound 2 within 5.0Å range:
|
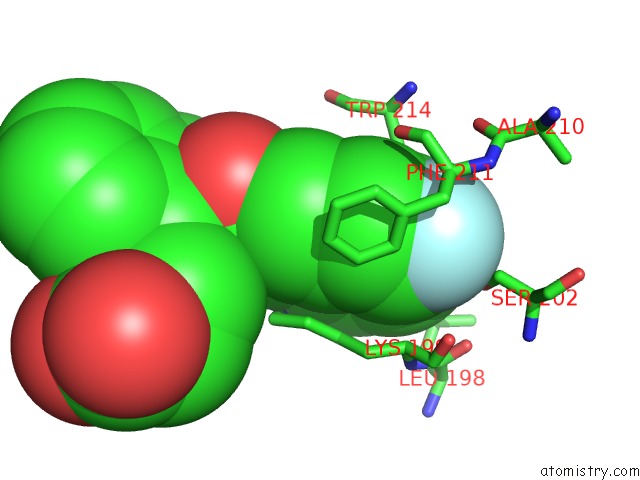
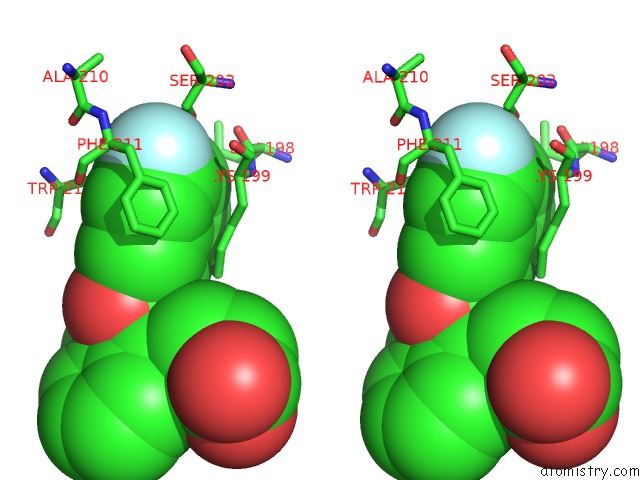
Fluorine binding site 2 out of 2 in 3lu7
Go back to
Fluorine binding site 2 out
of 2 in the Human Serum Albumin in Complex with Compound 2
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Human Serum Albumin in Complex with Compound 2 within 5.0Å range:
|
Reference:
D.Buttar,
N.Colclough,
S.Gerhardt,
P.A.Macfaul,
S.D.Phillips,
A.Plowright,
P.Whittamore,
K.Tam,
K.Maskos,
S.Steinbacher,
H.Steuber.
A Combined Spectroscopic and Crystallographic Approach to Probing Drug-Human Serum Albumin Interactions Bioorg.Med.Chem. V. 18 7486 2010.
ISSN: ISSN 0968-0896
PubMed: 20869876
DOI: 10.1016/J.BMC.2010.08.052
Page generated: Mon Jul 14 17:50:24 2025
ISSN: ISSN 0968-0896
PubMed: 20869876
DOI: 10.1016/J.BMC.2010.08.052
Last articles
K in 9ED0K in 9GEF
K in 9GBY
K in 9G9V
K in 9G9X
K in 9G9W
K in 9FDA
K in 9FCO
K in 9G5E
K in 9G5D