Fluorine »
PDB 5nk4-5o6j »
5nqe »
Fluorine in PDB 5nqe: Human PARP14 (ARTD8), Catalytic Fragment in Complex with An N-Aryl Piperazine Inhibitor
Enzymatic activity of Human PARP14 (ARTD8), Catalytic Fragment in Complex with An N-Aryl Piperazine Inhibitor
All present enzymatic activity of Human PARP14 (ARTD8), Catalytic Fragment in Complex with An N-Aryl Piperazine Inhibitor:
2.4.2.30;
2.4.2.30;
Protein crystallography data
The structure of Human PARP14 (ARTD8), Catalytic Fragment in Complex with An N-Aryl Piperazine Inhibitor, PDB code: 5nqe
was solved by
T.Karlberg,
A.G.Thorsell,
H.Schuler,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 45.18 / 2.71 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 83.430, 90.360, 80.070, 90.00, 116.42, 90.00 |
| R / Rfree (%) | 22.7 / 25.8 |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Human PARP14 (ARTD8), Catalytic Fragment in Complex with An N-Aryl Piperazine Inhibitor
(pdb code 5nqe). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 2 binding sites of Fluorine where determined in the Human PARP14 (ARTD8), Catalytic Fragment in Complex with An N-Aryl Piperazine Inhibitor, PDB code: 5nqe:
Jump to Fluorine binding site number: 1; 2;
In total 2 binding sites of Fluorine where determined in the Human PARP14 (ARTD8), Catalytic Fragment in Complex with An N-Aryl Piperazine Inhibitor, PDB code: 5nqe:
Jump to Fluorine binding site number: 1; 2;
Fluorine binding site 1 out of 2 in 5nqe
Go back to
Fluorine binding site 1 out
of 2 in the Human PARP14 (ARTD8), Catalytic Fragment in Complex with An N-Aryl Piperazine Inhibitor
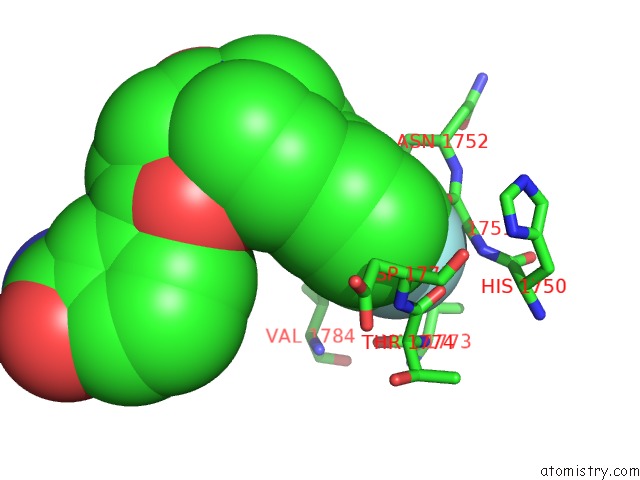
Mono view
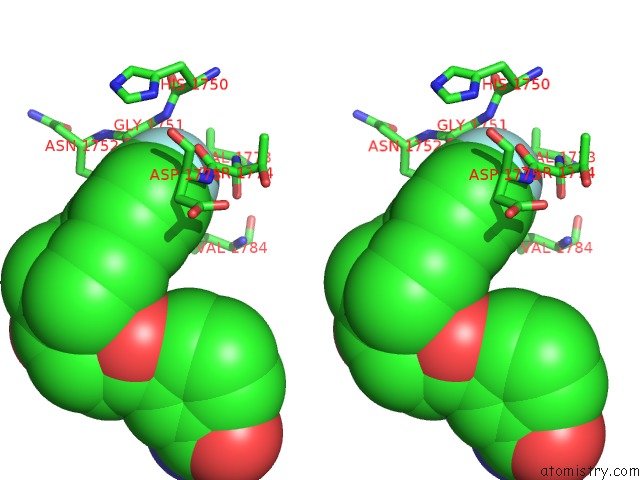
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Human PARP14 (ARTD8), Catalytic Fragment in Complex with An N-Aryl Piperazine Inhibitor within 5.0Å range:
|
Fluorine binding site 2 out of 2 in 5nqe
Go back to
Fluorine binding site 2 out
of 2 in the Human PARP14 (ARTD8), Catalytic Fragment in Complex with An N-Aryl Piperazine Inhibitor
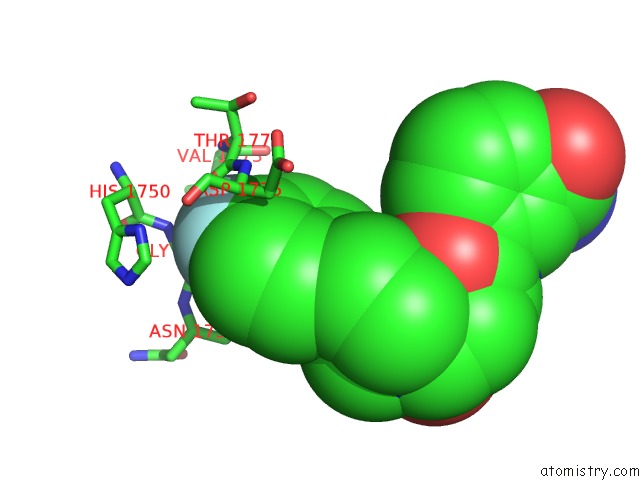
Mono view
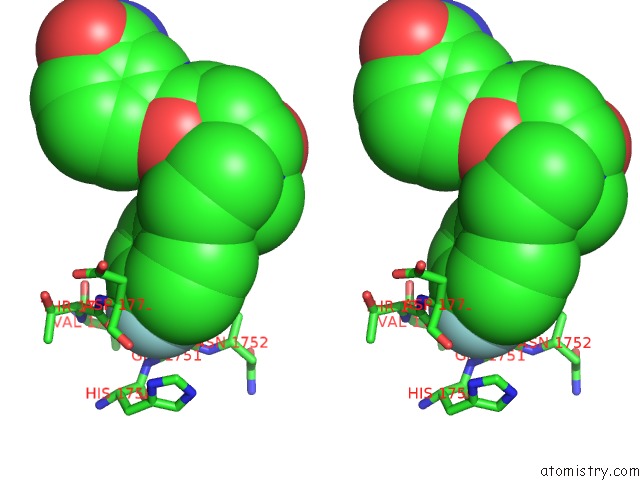
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Human PARP14 (ARTD8), Catalytic Fragment in Complex with An N-Aryl Piperazine Inhibitor within 5.0Å range:
|
Reference:
K.Upton,
M.Meyers,
A.G.Thorsell,
T.Karlberg,
J.Holechek,
R.Lease,
G.Schey,
E.Wolf,
A.Lucente,
H.Schuler,
D.Ferraris.
Design and Synthesis of Potent Inhibitors of the Mono(Adp-Ribosyl)Transferase, PARP14. Bioorg. Med. Chem. Lett. V. 27 2907 2017.
ISSN: ESSN 1464-3405
PubMed: 28495083
DOI: 10.1016/J.BMCL.2017.04.089
Page generated: Tue Jul 15 05:32:21 2025
ISSN: ESSN 1464-3405
PubMed: 28495083
DOI: 10.1016/J.BMCL.2017.04.089
Last articles
Na in 3HUPNa in 3HTL
Na in 3HSC
Na in 3HPT
Na in 3HLT
Na in 3HJZ
Na in 3HLJ
Na in 3HK7
Na in 3HKV
Na in 3HJB