Fluorine »
PDB 5tw3-5ug8 »
5u45 »
Fluorine in PDB 5u45: Human Ppardelta Ligand-Binding Domain in Complexed with Specific Agonist 14
Protein crystallography data
The structure of Human Ppardelta Ligand-Binding Domain in Complexed with Specific Agonist 14, PDB code: 5u45
was solved by
C.-C.Wu,
T.J.Baiga,
M.Downes,
J.J.La Clair,
A.R.Atkins,
S.B.Richard,
T.A.Stockley-Noel,
M.E.Bowman,
R.M.Evans,
J.P.Noel,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 47.80 / 1.95 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 39.530, 95.260, 96.440, 90.00, 97.56, 90.00 |
| R / Rfree (%) | 19 / 24.1 |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Human Ppardelta Ligand-Binding Domain in Complexed with Specific Agonist 14
(pdb code 5u45). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 2 binding sites of Fluorine where determined in the Human Ppardelta Ligand-Binding Domain in Complexed with Specific Agonist 14, PDB code: 5u45:
Jump to Fluorine binding site number: 1; 2;
In total 2 binding sites of Fluorine where determined in the Human Ppardelta Ligand-Binding Domain in Complexed with Specific Agonist 14, PDB code: 5u45:
Jump to Fluorine binding site number: 1; 2;
Fluorine binding site 1 out of 2 in 5u45
Go back to
Fluorine binding site 1 out
of 2 in the Human Ppardelta Ligand-Binding Domain in Complexed with Specific Agonist 14
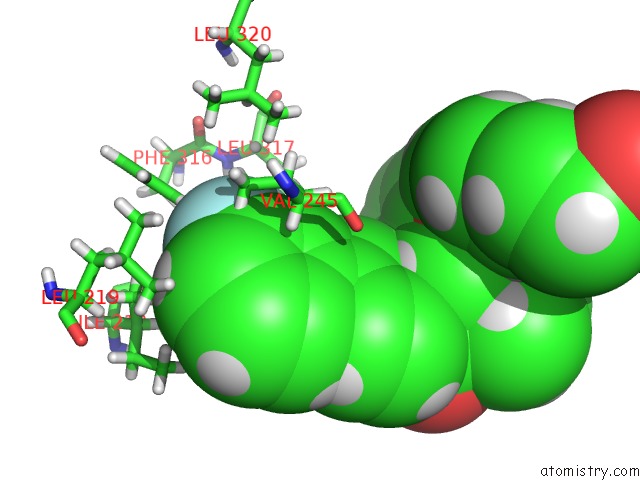
Mono view
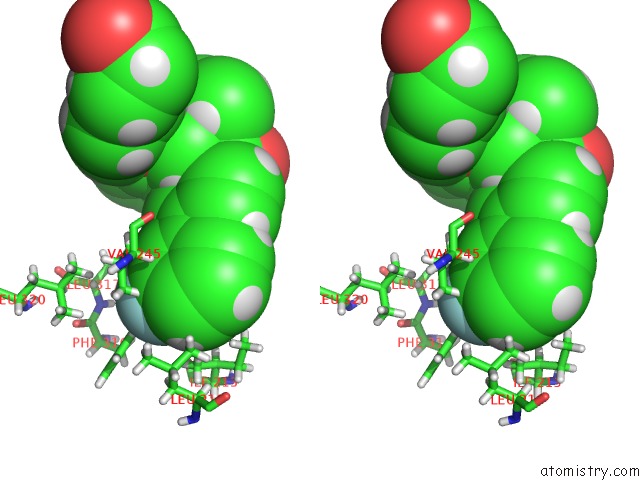
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Human Ppardelta Ligand-Binding Domain in Complexed with Specific Agonist 14 within 5.0Å range:
|
Fluorine binding site 2 out of 2 in 5u45
Go back to
Fluorine binding site 2 out
of 2 in the Human Ppardelta Ligand-Binding Domain in Complexed with Specific Agonist 14
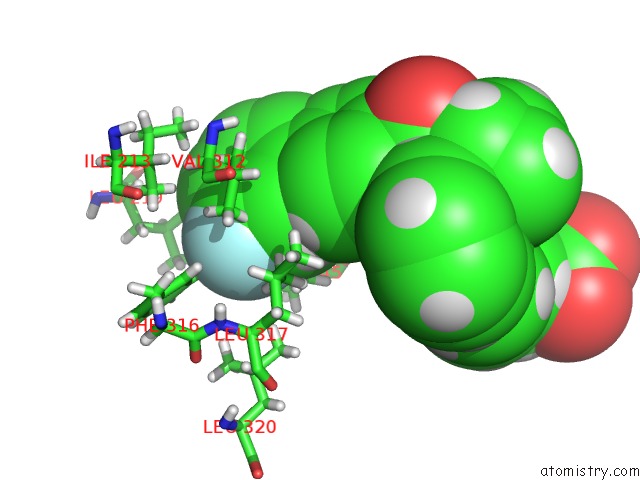
Mono view
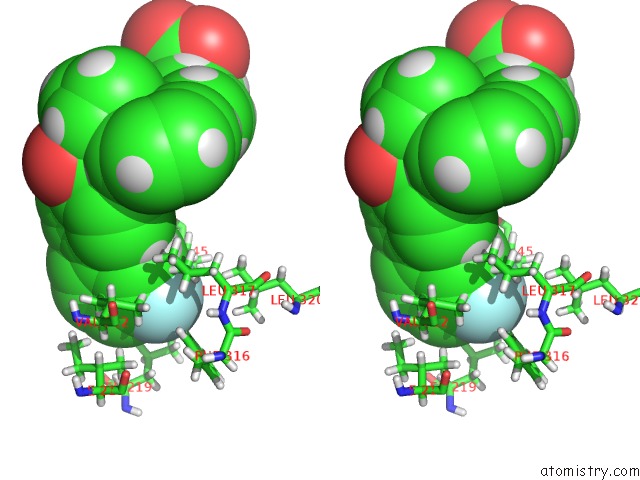
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Human Ppardelta Ligand-Binding Domain in Complexed with Specific Agonist 14 within 5.0Å range:
|
Reference:
C.C.Wu,
T.J.Baiga,
M.Downes,
J.J.La Clair,
A.R.Atkins,
S.B.Richard,
W.Fan,
T.A.Stockley-Noel,
M.E.Bowman,
J.P.Noel,
R.M.Evans.
Structural Basis For Specific Ligation of the Peroxisome Proliferator-Activated Receptor Delta. Proc. Natl. Acad. Sci. V. 114 E2563 2017U.S.A..
ISSN: ESSN 1091-6490
PubMed: 28320959
DOI: 10.1073/PNAS.1621513114
Page generated: Tue Jul 15 08:10:03 2025
ISSN: ESSN 1091-6490
PubMed: 28320959
DOI: 10.1073/PNAS.1621513114
Last articles
Fe in 8XLSFe in 8XLP
Fe in 8XMQ
Fe in 8XMP
Fe in 8XIW
Fe in 8XCM
Fe in 8XCN
Fe in 8XHT
Fe in 8XHP
Fe in 8XFI