Fluorine »
PDB 8g63-8gky »
8gku »
Fluorine in PDB 8gku: Human Mitochondrial Serine Hydroxymethyltransferase (SHMT2) in Complex with Plp, Glycine and AGF355 Inhibitor
Enzymatic activity of Human Mitochondrial Serine Hydroxymethyltransferase (SHMT2) in Complex with Plp, Glycine and AGF355 Inhibitor
All present enzymatic activity of Human Mitochondrial Serine Hydroxymethyltransferase (SHMT2) in Complex with Plp, Glycine and AGF355 Inhibitor:
2.1.2.1;
2.1.2.1;
Protein crystallography data
The structure of Human Mitochondrial Serine Hydroxymethyltransferase (SHMT2) in Complex with Plp, Glycine and AGF355 Inhibitor, PDB code: 8gku
was solved by
J.M.Katinas,
C.E.Dann Iii,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 48.71 / 3.06 |
| Space group | P 65 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 158.324, 158.324, 207.65, 90, 90, 120 |
| R / Rfree (%) | 21.3 / 26.4 |
Fluorine Binding Sites:
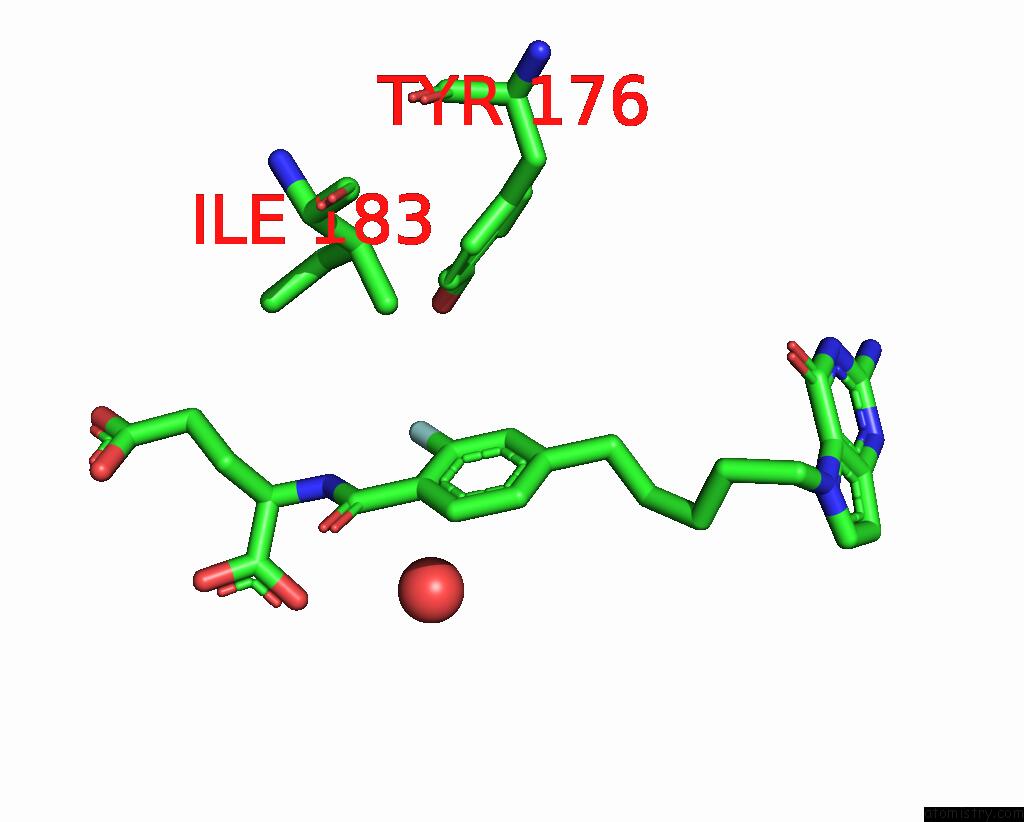
The binding sites of Fluorine atom in the Human Mitochondrial Serine Hydroxymethyltransferase (SHMT2) in Complex with Plp, Glycine and AGF355 Inhibitor
(pdb code 8gku). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total only one binding site of Fluorine was determined in the Human Mitochondrial Serine Hydroxymethyltransferase (SHMT2) in Complex with Plp, Glycine and AGF355 Inhibitor, PDB code: 8gku:
In total only one binding site of Fluorine was determined in the Human Mitochondrial Serine Hydroxymethyltransferase (SHMT2) in Complex with Plp, Glycine and AGF355 Inhibitor, PDB code: 8gku:
Fluorine binding site 1 out of 1 in 8gku
Go back to
Fluorine binding site 1 out
of 1 in the Human Mitochondrial Serine Hydroxymethyltransferase (SHMT2) in Complex with Plp, Glycine and AGF355 Inhibitor
Mono view
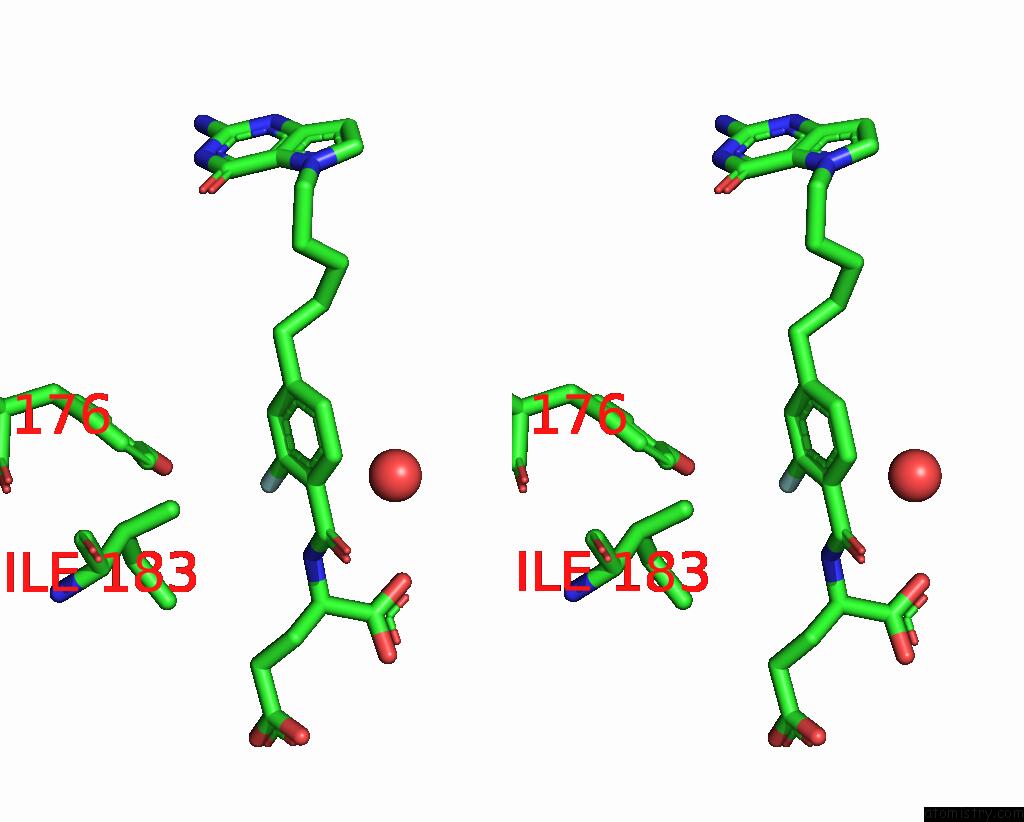
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Human Mitochondrial Serine Hydroxymethyltransferase (SHMT2) in Complex with Plp, Glycine and AGF355 Inhibitor within 5.0Å range:
|
Reference:
J.M.Katinas,
M.J.Nayeen,
M.Schneider,
K.Shah,
A.N.Fifer,
L.M.Klapper,
A.Sharma,
K.Thalluri,
M.S.Van Nieuwenhze,
Z.Hou,
A.Gangjee,
L.H.Matherly,
C.E.Dann 3Rd.
Structural Characterization of 5-Substituted Pyrrolo[3,2- D ]Pyrimidine Antifolate Inhibitors in Complex with Human Serine Hydroxymethyl Transferase 2. Biochemistry 2024.
ISSN: ISSN 0006-2960
PubMed: 38324671
DOI: 10.1021/ACS.BIOCHEM.3C00613
Page generated: Wed Jul 16 05:06:15 2025
ISSN: ISSN 0006-2960
PubMed: 38324671
DOI: 10.1021/ACS.BIOCHEM.3C00613
Last articles
Mn in 5WF3Mn in 5WEY
Mn in 5WEI
Mn in 5WEB
Mn in 5WEF
Mn in 5WDY
Mn in 5WE8
Mn in 5WE7
Mn in 5WE9
Mn in 5WDW