Fluorine »
PDB 16pk-1bw7 »
1bjg »
Fluorine in PDB 1bjg: D221(169)N Mutant Does Not Promote Opening of the Cofactor Imidazolidine Ring
Enzymatic activity of D221(169)N Mutant Does Not Promote Opening of the Cofactor Imidazolidine Ring
All present enzymatic activity of D221(169)N Mutant Does Not Promote Opening of the Cofactor Imidazolidine Ring:
2.1.1.45;
2.1.1.45;
Protein crystallography data
The structure of D221(169)N Mutant Does Not Promote Opening of the Cofactor Imidazolidine Ring, PDB code: 1bjg
was solved by
C.R.Sage,
M.D.Michelitsch,
J.Finer-Moore,
R.M.Stroud,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 7.00 / 2.30 |
| Space group | I 21 3 |
| Cell size a, b, c (Å), α, β, γ (°) | 133.000, 133.000, 133.000, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 19 / 23.4 |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the D221(169)N Mutant Does Not Promote Opening of the Cofactor Imidazolidine Ring
(pdb code 1bjg). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total only one binding site of Fluorine was determined in the D221(169)N Mutant Does Not Promote Opening of the Cofactor Imidazolidine Ring, PDB code: 1bjg:
In total only one binding site of Fluorine was determined in the D221(169)N Mutant Does Not Promote Opening of the Cofactor Imidazolidine Ring, PDB code: 1bjg:
Fluorine binding site 1 out of 1 in 1bjg
Go back to
Fluorine binding site 1 out
of 1 in the D221(169)N Mutant Does Not Promote Opening of the Cofactor Imidazolidine Ring
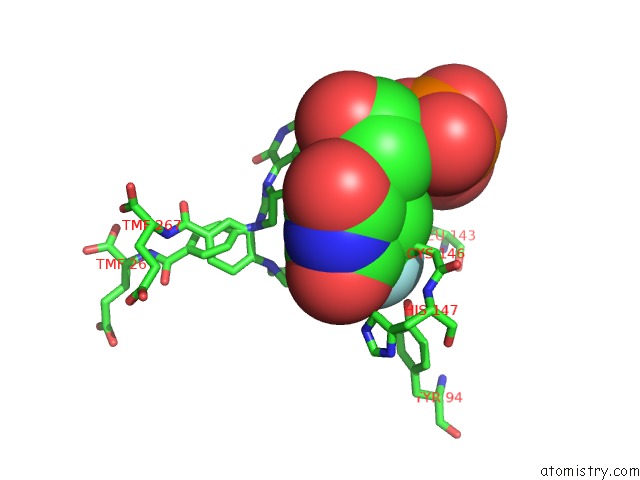
Mono view
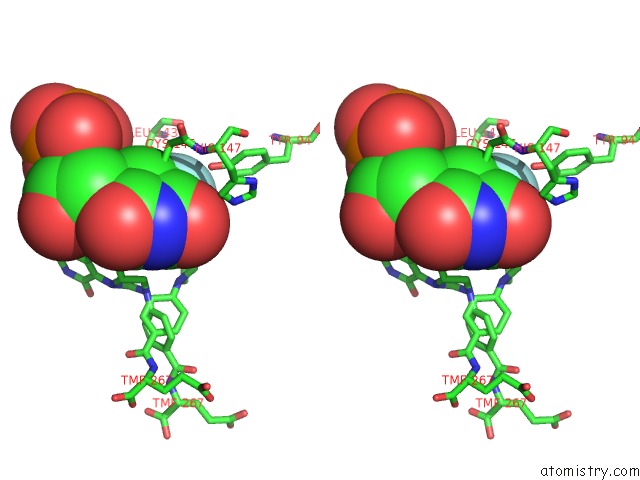
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of D221(169)N Mutant Does Not Promote Opening of the Cofactor Imidazolidine Ring within 5.0Å range:
|
Reference:
C.R.Sage,
M.D.Michelitsch,
T.J.Stout,
D.Biermann,
R.Nissen,
J.Finer-Moore,
R.M.Stroud.
D221 in Thymidylate Synthase Controls Conformation Change, and Thereby Opening of the Imidazolidine. Biochemistry V. 37 13893 1998.
ISSN: ISSN 0006-2960
PubMed: 9753479
DOI: 10.1021/BI9810510
Page generated: Wed Jul 31 10:50:30 2024
ISSN: ISSN 0006-2960
PubMed: 9753479
DOI: 10.1021/BI9810510
Last articles
Zn in 9JYWZn in 9IR4
Zn in 9IR3
Zn in 9GMX
Zn in 9GMW
Zn in 9JEJ
Zn in 9ERF
Zn in 9ERE
Zn in 9EGV
Zn in 9EGW