Fluorine »
PDB 1jdj-1mkd »
1kk8 »
Fluorine in PDB 1kk8: Scallop Myosin (S1-Adp-Befx) in the Actin-Detached Conformation
Protein crystallography data
The structure of Scallop Myosin (S1-Adp-Befx) in the Actin-Detached Conformation, PDB code: 1kk8
was solved by
M.Himmel,
S.Gourinath,
L.Reshetnikova,
Y.Shen,
G.Szent-Gyorgyi,
C.Cohen,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.84 / 2.30 |
| Space group | P 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 51.604, 58.527, 133.295, 81.08, 84.94, 67.24 |
| R / Rfree (%) | 23 / 26.9 |
Other elements in 1kk8:
The structure of Scallop Myosin (S1-Adp-Befx) in the Actin-Detached Conformation also contains other interesting chemical elements:
| Magnesium | (Mg) | 2 atoms |
| Calcium | (Ca) | 1 atom |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Scallop Myosin (S1-Adp-Befx) in the Actin-Detached Conformation
(pdb code 1kk8). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 3 binding sites of Fluorine where determined in the Scallop Myosin (S1-Adp-Befx) in the Actin-Detached Conformation, PDB code: 1kk8:
Jump to Fluorine binding site number: 1; 2; 3;
In total 3 binding sites of Fluorine where determined in the Scallop Myosin (S1-Adp-Befx) in the Actin-Detached Conformation, PDB code: 1kk8:
Jump to Fluorine binding site number: 1; 2; 3;
Fluorine binding site 1 out of 3 in 1kk8
Go back to
Fluorine binding site 1 out
of 3 in the Scallop Myosin (S1-Adp-Befx) in the Actin-Detached Conformation
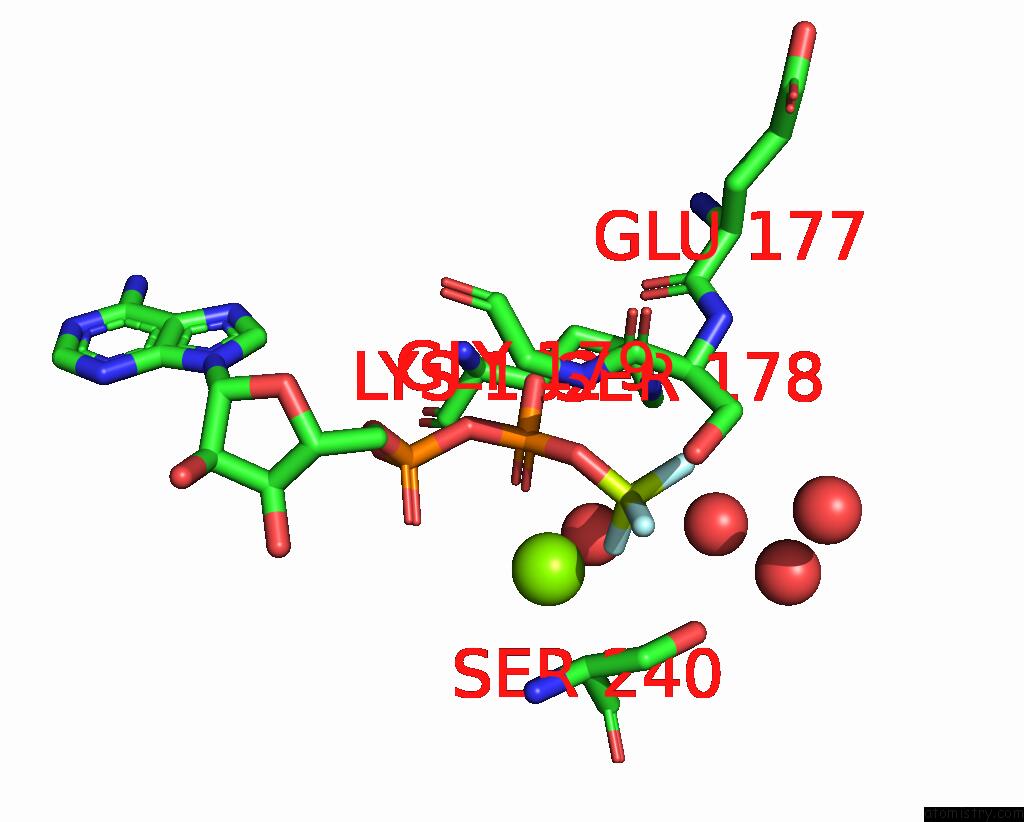
Mono view
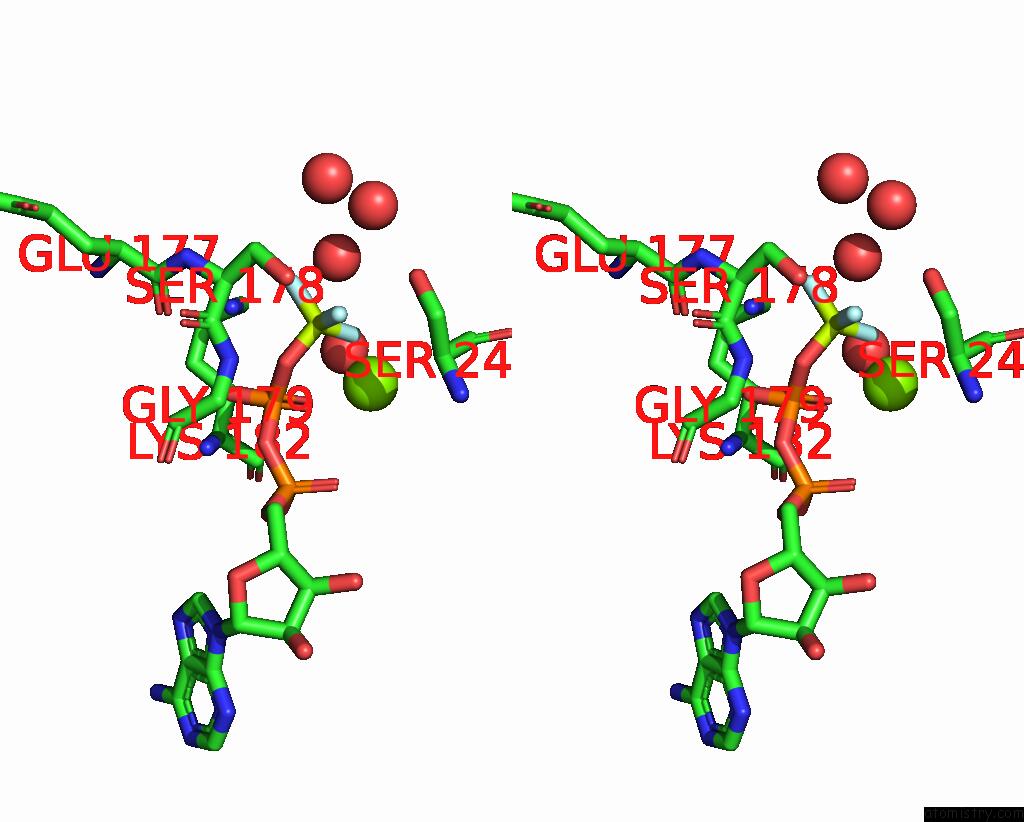
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Scallop Myosin (S1-Adp-Befx) in the Actin-Detached Conformation within 5.0Å range:
|
Fluorine binding site 2 out of 3 in 1kk8
Go back to
Fluorine binding site 2 out
of 3 in the Scallop Myosin (S1-Adp-Befx) in the Actin-Detached Conformation
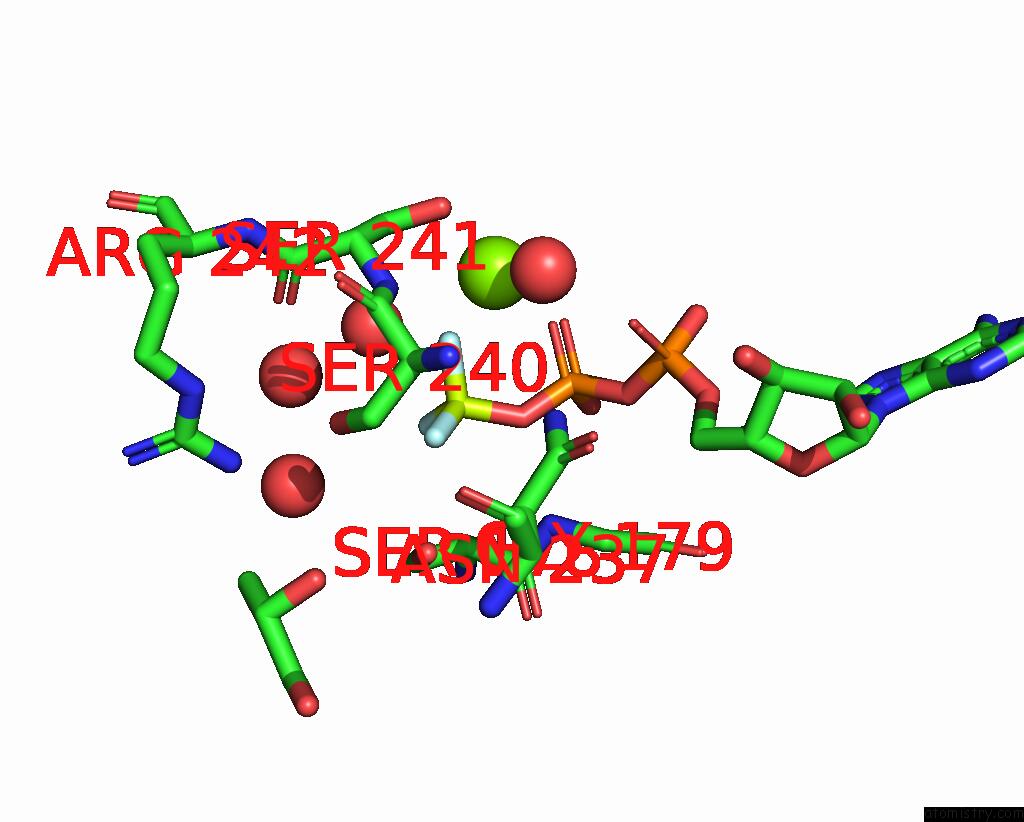
Mono view
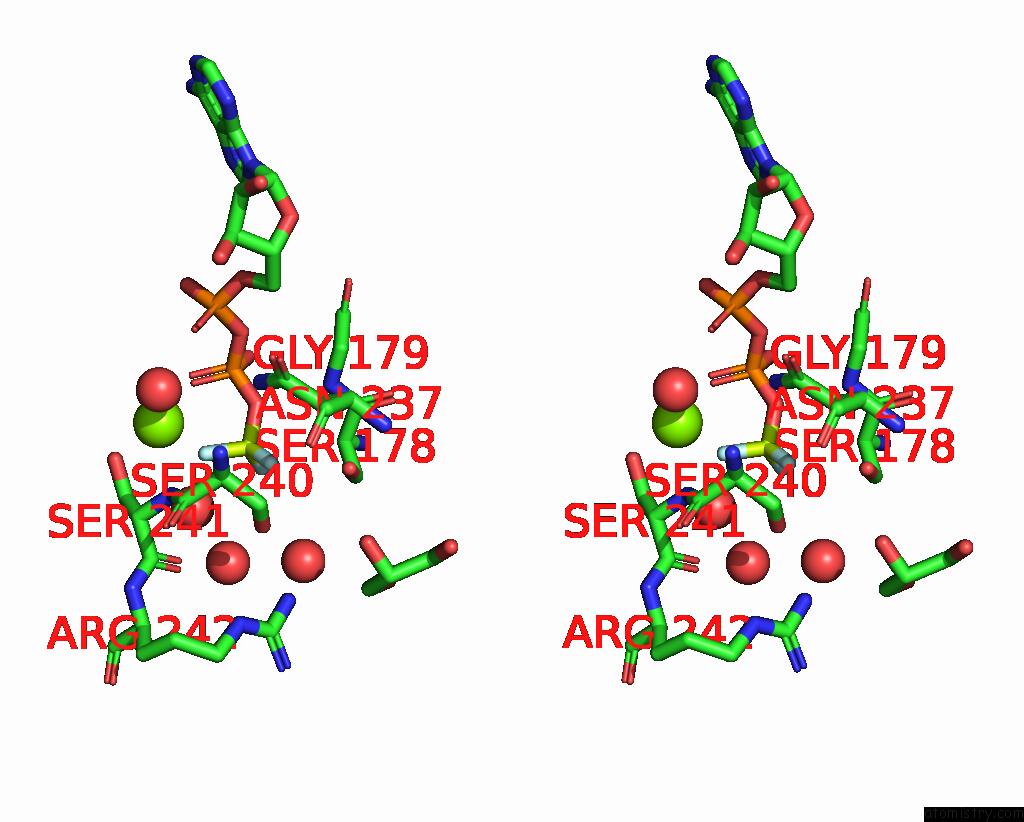
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Scallop Myosin (S1-Adp-Befx) in the Actin-Detached Conformation within 5.0Å range:
|
Fluorine binding site 3 out of 3 in 1kk8
Go back to
Fluorine binding site 3 out
of 3 in the Scallop Myosin (S1-Adp-Befx) in the Actin-Detached Conformation
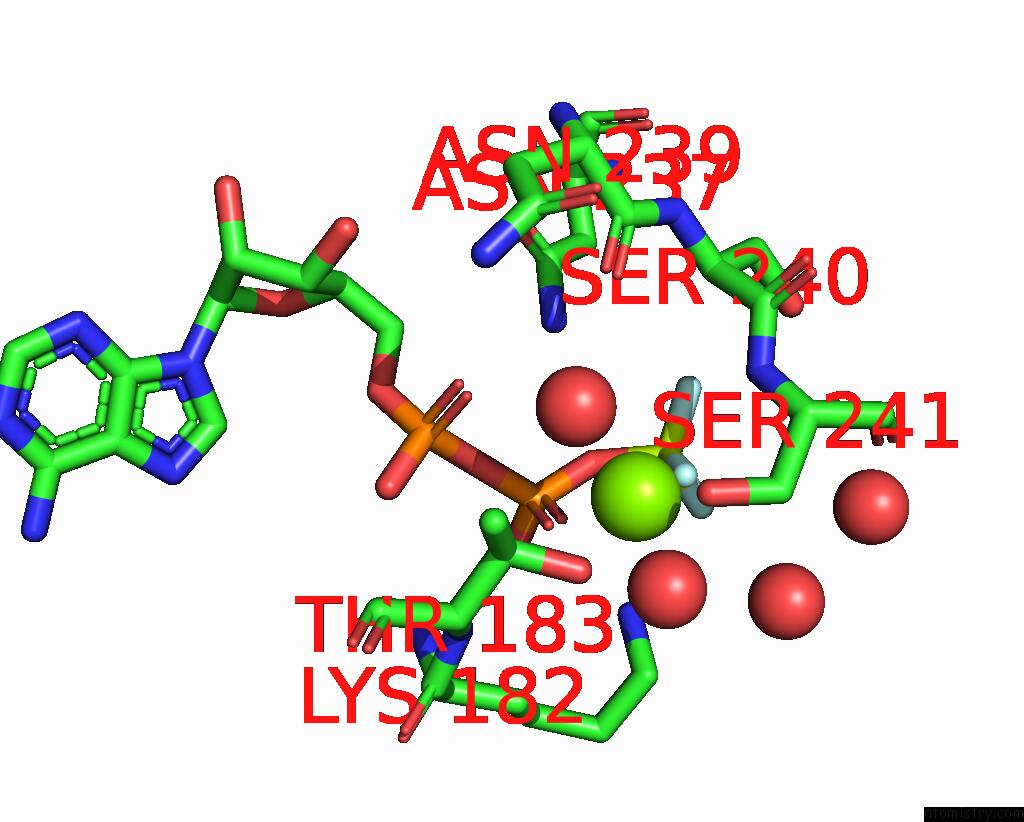
Mono view
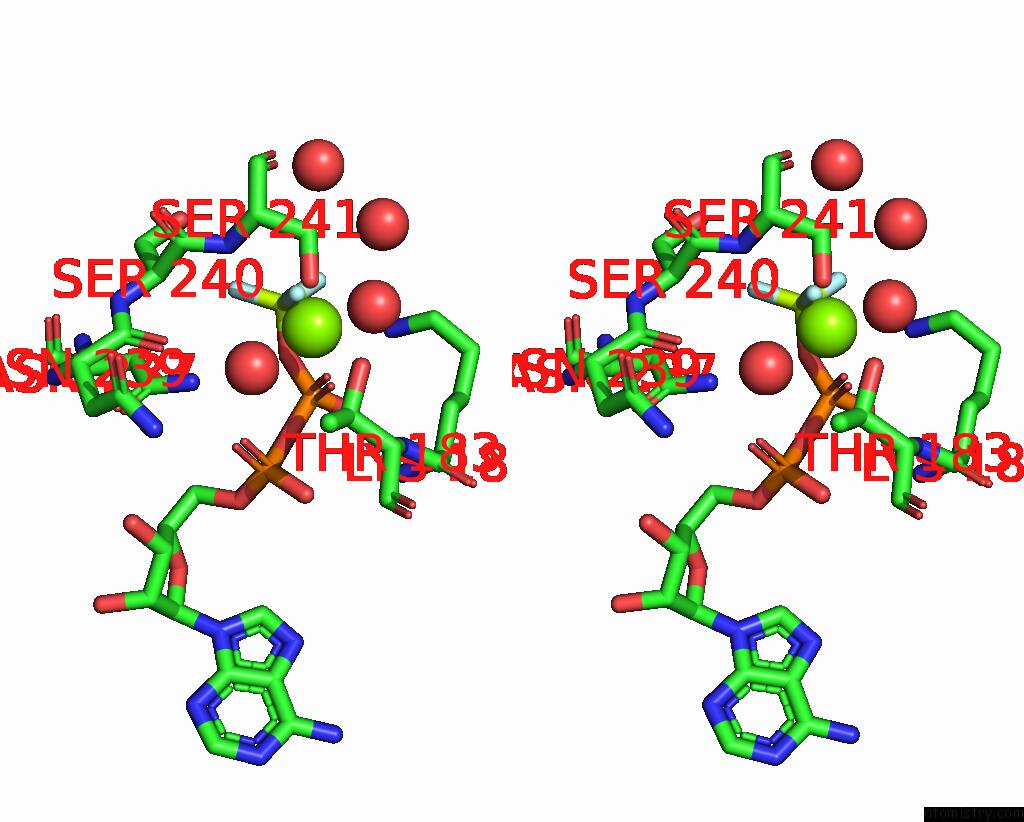
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Scallop Myosin (S1-Adp-Befx) in the Actin-Detached Conformation within 5.0Å range:
|
Reference:
D.M.Himmel,
S.Gourinath,
L.Reshetnikova,
Y.Shen,
A.G.Szent-Gyorgyi,
C.Cohen.
Crystallographic Findings on the Internally Uncoupled and Near-Rigor States of Myosin: Further Insights Into the Mechanics of the Motor. Proc.Natl.Acad.Sci.Usa V. 99 12645 2002.
ISSN: ISSN 0027-8424
PubMed: 12297624
DOI: 10.1073/PNAS.202476799
Page generated: Wed Jul 31 11:44:11 2024
ISSN: ISSN 0027-8424
PubMed: 12297624
DOI: 10.1073/PNAS.202476799
Last articles
Ca in 5XQKCa in 5XNP
Ca in 5XQA
Ca in 5XQ3
Ca in 5XPX
Ca in 5XP3
Ca in 5XPS
Ca in 5XNQ
Ca in 5XNR
Ca in 5XNM