Fluorine »
PDB 2gtn-2ihj »
2h9y »
Fluorine in PDB 2h9y: Crystal Structure of Mouse Acetylcholinesterase Complexed with M-(N,N, N-Trimethylammonio)Trifluoroacetophenone
Enzymatic activity of Crystal Structure of Mouse Acetylcholinesterase Complexed with M-(N,N, N-Trimethylammonio)Trifluoroacetophenone
All present enzymatic activity of Crystal Structure of Mouse Acetylcholinesterase Complexed with M-(N,N, N-Trimethylammonio)Trifluoroacetophenone:
3.1.1.7;
3.1.1.7;
Protein crystallography data
The structure of Crystal Structure of Mouse Acetylcholinesterase Complexed with M-(N,N, N-Trimethylammonio)Trifluoroacetophenone, PDB code: 2h9y
was solved by
Y.Bourne,
Z.Radic,
G.Sulzenbacher,
E.Kim,
P.Taylor,
P.Marchot,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 20.00 / 2.40 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 79.013, 111.394, 226.934, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 18.6 / 21.4 |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Crystal Structure of Mouse Acetylcholinesterase Complexed with M-(N,N, N-Trimethylammonio)Trifluoroacetophenone
(pdb code 2h9y). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 6 binding sites of Fluorine where determined in the Crystal Structure of Mouse Acetylcholinesterase Complexed with M-(N,N, N-Trimethylammonio)Trifluoroacetophenone, PDB code: 2h9y:
Jump to Fluorine binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Fluorine where determined in the Crystal Structure of Mouse Acetylcholinesterase Complexed with M-(N,N, N-Trimethylammonio)Trifluoroacetophenone, PDB code: 2h9y:
Jump to Fluorine binding site number: 1; 2; 3; 4; 5; 6;
Fluorine binding site 1 out of 6 in 2h9y
Go back to
Fluorine binding site 1 out
of 6 in the Crystal Structure of Mouse Acetylcholinesterase Complexed with M-(N,N, N-Trimethylammonio)Trifluoroacetophenone
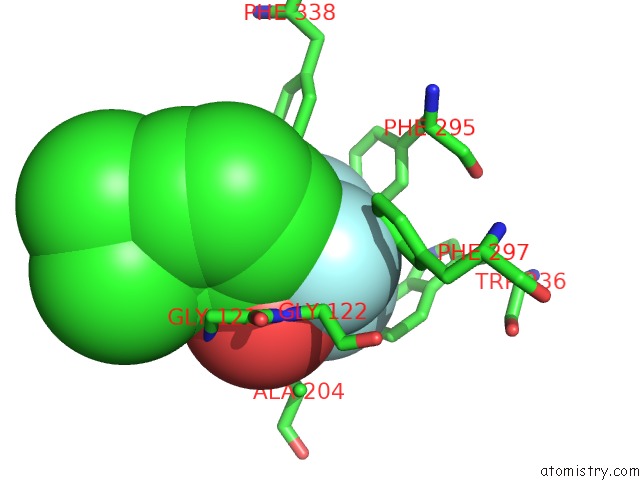
Mono view
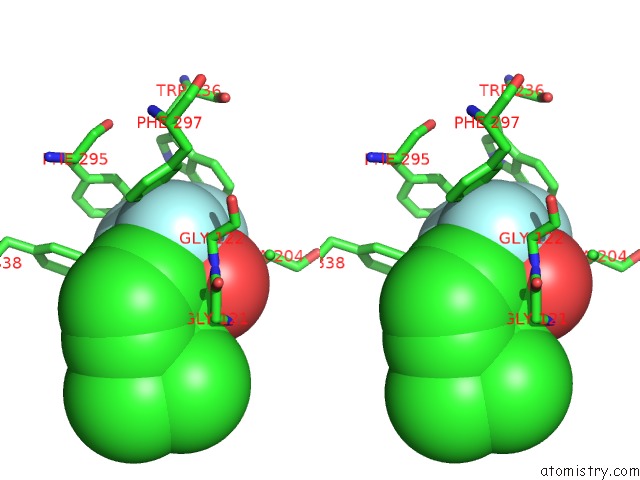
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Crystal Structure of Mouse Acetylcholinesterase Complexed with M-(N,N, N-Trimethylammonio)Trifluoroacetophenone within 5.0Å range:
|
Fluorine binding site 2 out of 6 in 2h9y
Go back to
Fluorine binding site 2 out
of 6 in the Crystal Structure of Mouse Acetylcholinesterase Complexed with M-(N,N, N-Trimethylammonio)Trifluoroacetophenone
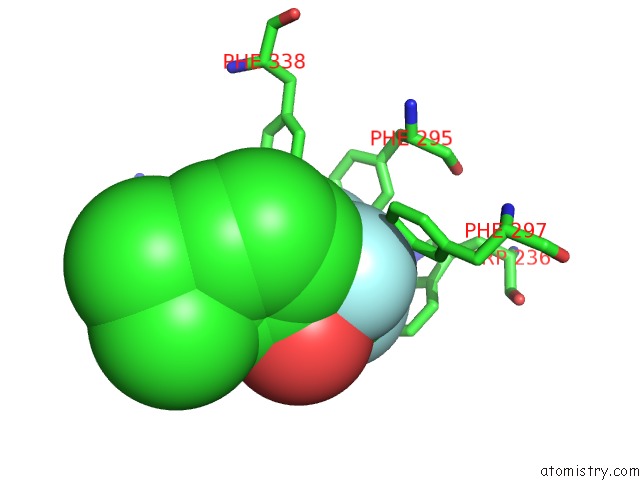
Mono view
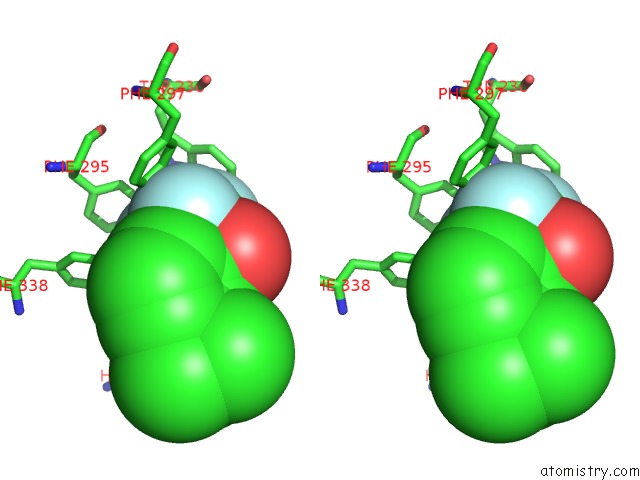
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Crystal Structure of Mouse Acetylcholinesterase Complexed with M-(N,N, N-Trimethylammonio)Trifluoroacetophenone within 5.0Å range:
|
Fluorine binding site 3 out of 6 in 2h9y
Go back to
Fluorine binding site 3 out
of 6 in the Crystal Structure of Mouse Acetylcholinesterase Complexed with M-(N,N, N-Trimethylammonio)Trifluoroacetophenone
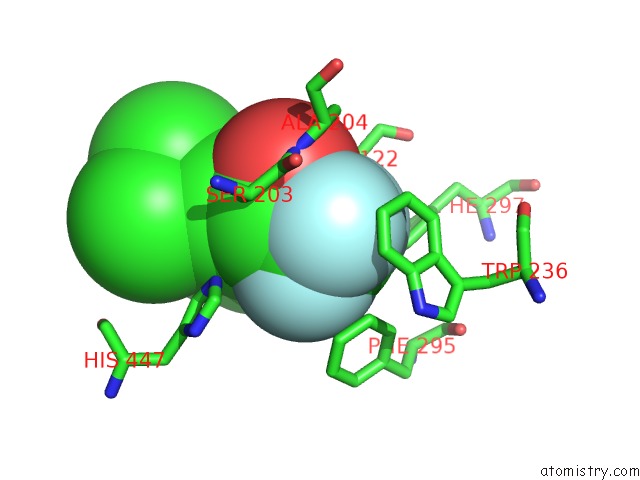
Mono view
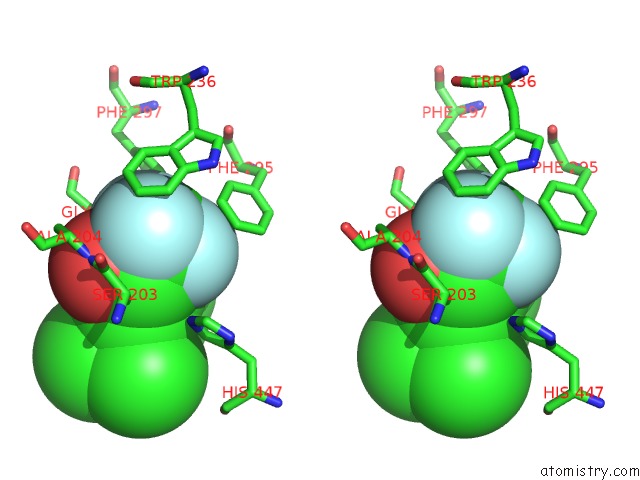
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Crystal Structure of Mouse Acetylcholinesterase Complexed with M-(N,N, N-Trimethylammonio)Trifluoroacetophenone within 5.0Å range:
|
Fluorine binding site 4 out of 6 in 2h9y
Go back to
Fluorine binding site 4 out
of 6 in the Crystal Structure of Mouse Acetylcholinesterase Complexed with M-(N,N, N-Trimethylammonio)Trifluoroacetophenone
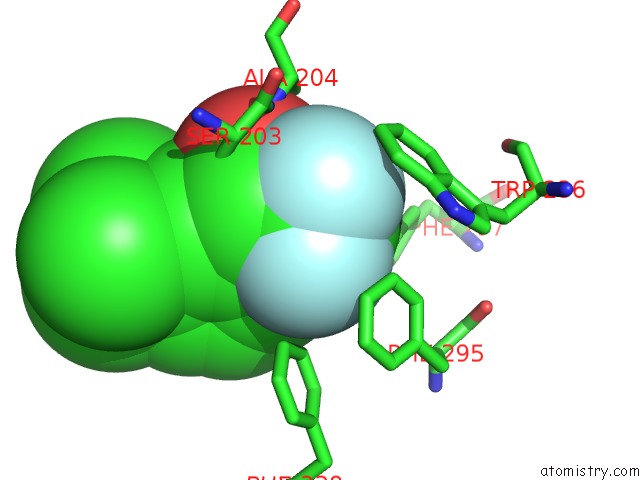
Mono view
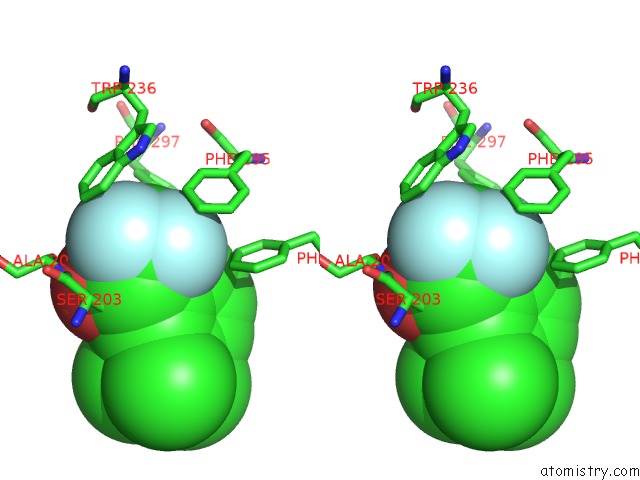
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 4 of Crystal Structure of Mouse Acetylcholinesterase Complexed with M-(N,N, N-Trimethylammonio)Trifluoroacetophenone within 5.0Å range:
|
Fluorine binding site 5 out of 6 in 2h9y
Go back to
Fluorine binding site 5 out
of 6 in the Crystal Structure of Mouse Acetylcholinesterase Complexed with M-(N,N, N-Trimethylammonio)Trifluoroacetophenone
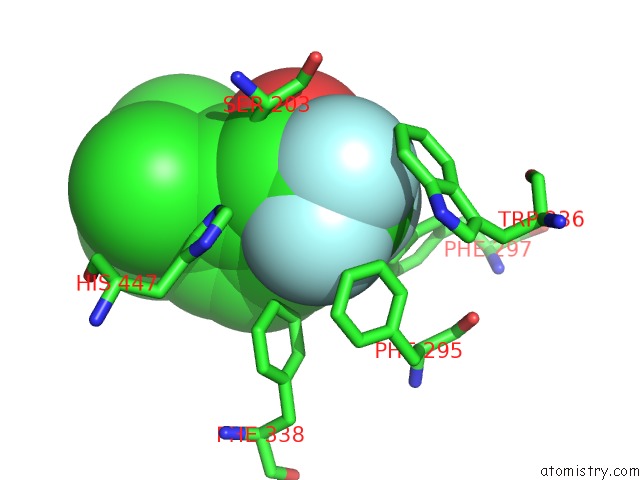
Mono view
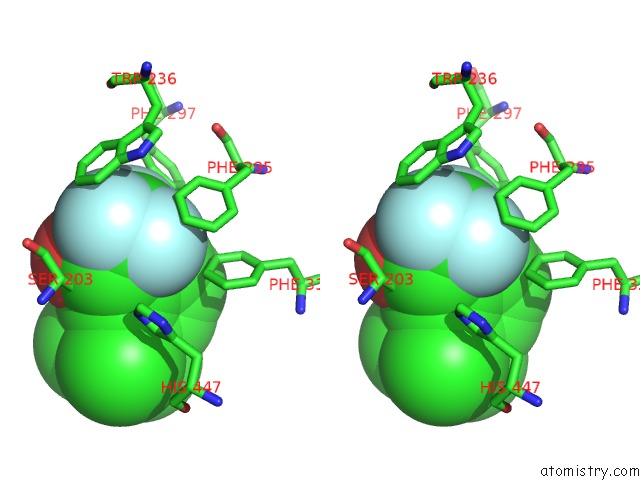
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 5 of Crystal Structure of Mouse Acetylcholinesterase Complexed with M-(N,N, N-Trimethylammonio)Trifluoroacetophenone within 5.0Å range:
|
Fluorine binding site 6 out of 6 in 2h9y
Go back to
Fluorine binding site 6 out
of 6 in the Crystal Structure of Mouse Acetylcholinesterase Complexed with M-(N,N, N-Trimethylammonio)Trifluoroacetophenone
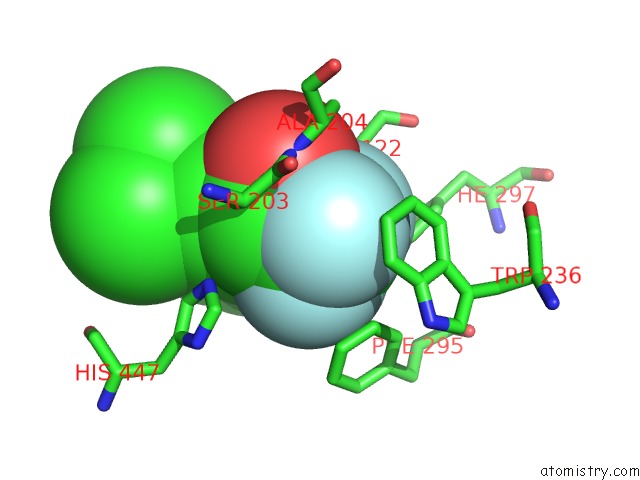
Mono view
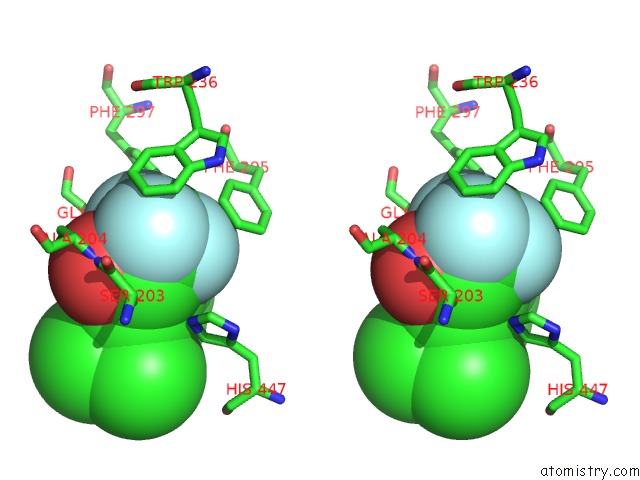
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 6 of Crystal Structure of Mouse Acetylcholinesterase Complexed with M-(N,N, N-Trimethylammonio)Trifluoroacetophenone within 5.0Å range:
|
Reference:
Y.Bourne,
Z.Radic,
G.Sulzenbacher,
E.Kim,
P.Taylor,
P.Marchot.
Substrate and Product Trafficking Through the Active Center Gorge of Acetylcholinesterase Analyzed By Crystallography and Equilibrium Binding J.Biol.Chem. V. 281 29256 2006.
ISSN: ISSN 0021-9258
PubMed: 16837465
DOI: 10.1074/JBC.M603018200
Page generated: Wed Jul 31 14:41:45 2024
ISSN: ISSN 0021-9258
PubMed: 16837465
DOI: 10.1074/JBC.M603018200
Last articles
Cl in 5U0LCl in 5U0I
Cl in 5TZX
Cl in 5TZO
Cl in 5U09
Cl in 5TY2
Cl in 5TZL
Cl in 5TYK
Cl in 5TWS
Cl in 5TXU