Fluorine »
PDB 2rfn-2vfz »
2v0n »
Fluorine in PDB 2v0n: Activated Response Regulator Pled in Complex with C-Digmp and Gtp- Alpha-S
Protein crystallography data
The structure of Activated Response Regulator Pled in Complex with C-Digmp and Gtp- Alpha-S, PDB code: 2v0n
was solved by
P.Wassmann,
T.Schirmer,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 30.00 / 2.71 |
| Space group | P 21 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 128.965, 132.557, 88.425, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 21.5 / 25.2 |
Other elements in 2v0n:
The structure of Activated Response Regulator Pled in Complex with C-Digmp and Gtp- Alpha-S also contains other interesting chemical elements:
| Magnesium | (Mg) | 5 atoms |
| Chlorine | (Cl) | 1 atom |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Activated Response Regulator Pled in Complex with C-Digmp and Gtp- Alpha-S
(pdb code 2v0n). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 6 binding sites of Fluorine where determined in the Activated Response Regulator Pled in Complex with C-Digmp and Gtp- Alpha-S, PDB code: 2v0n:
Jump to Fluorine binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Fluorine where determined in the Activated Response Regulator Pled in Complex with C-Digmp and Gtp- Alpha-S, PDB code: 2v0n:
Jump to Fluorine binding site number: 1; 2; 3; 4; 5; 6;
Fluorine binding site 1 out of 6 in 2v0n
Go back to
Fluorine binding site 1 out
of 6 in the Activated Response Regulator Pled in Complex with C-Digmp and Gtp- Alpha-S
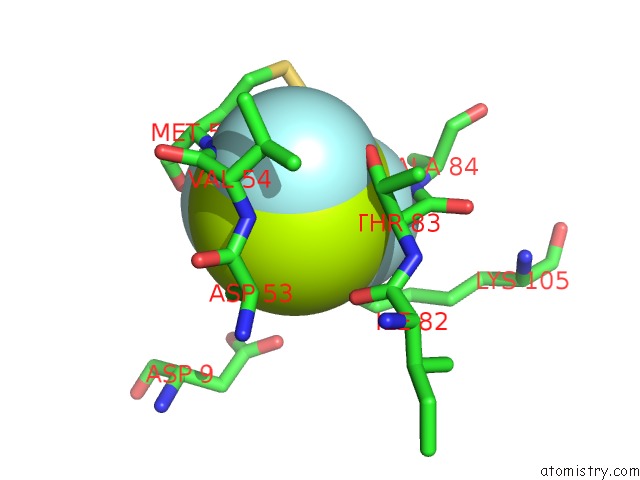
Mono view
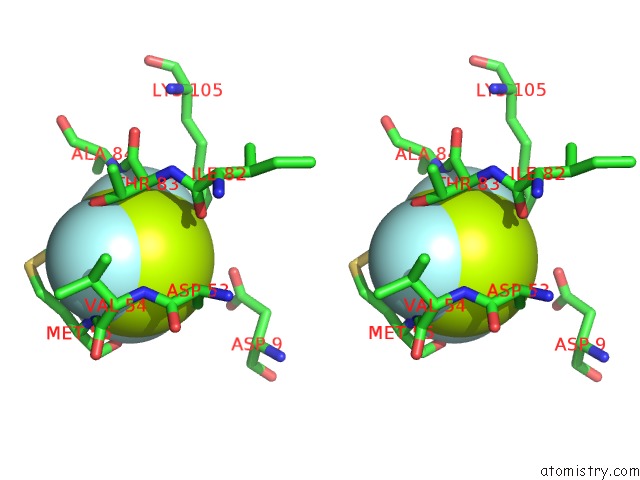
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Activated Response Regulator Pled in Complex with C-Digmp and Gtp- Alpha-S within 5.0Å range:
|
Fluorine binding site 2 out of 6 in 2v0n
Go back to
Fluorine binding site 2 out
of 6 in the Activated Response Regulator Pled in Complex with C-Digmp and Gtp- Alpha-S
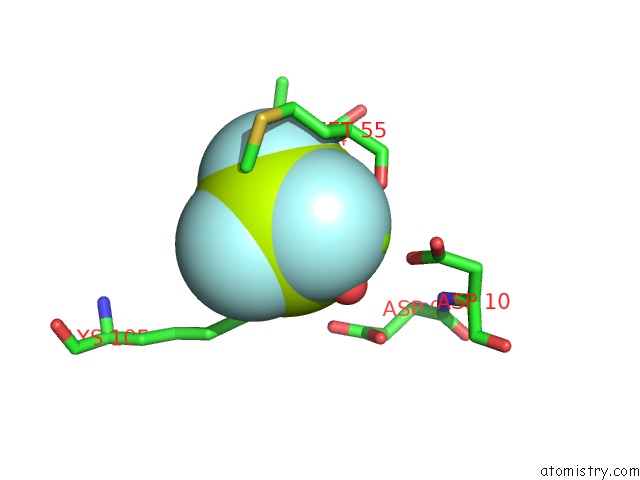
Mono view
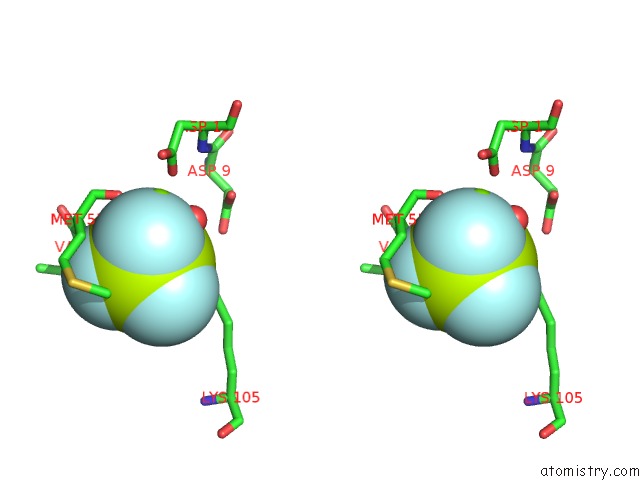
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Activated Response Regulator Pled in Complex with C-Digmp and Gtp- Alpha-S within 5.0Å range:
|
Fluorine binding site 3 out of 6 in 2v0n
Go back to
Fluorine binding site 3 out
of 6 in the Activated Response Regulator Pled in Complex with C-Digmp and Gtp- Alpha-S
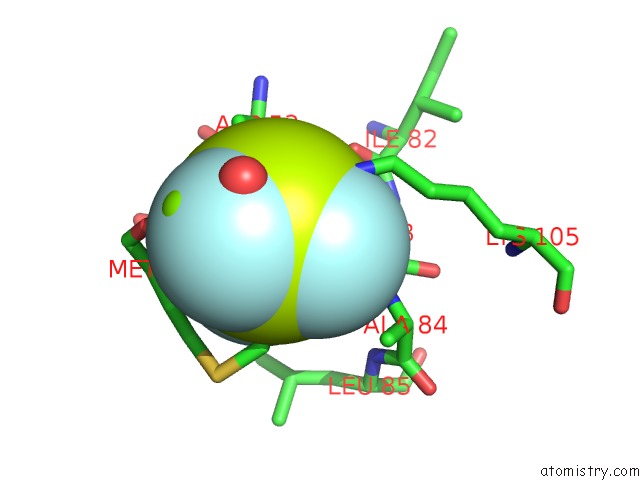
Mono view
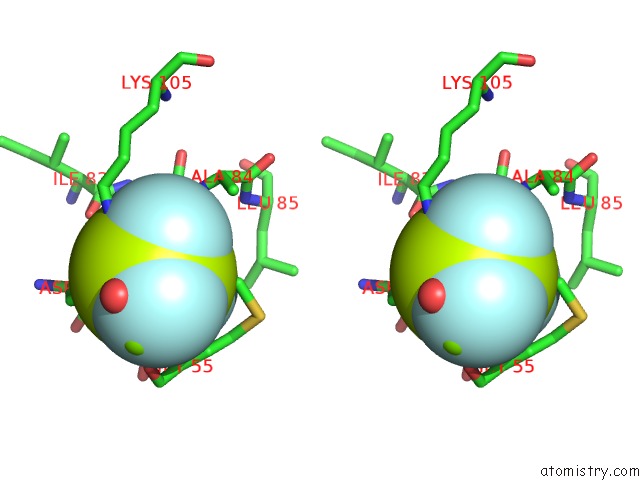
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Activated Response Regulator Pled in Complex with C-Digmp and Gtp- Alpha-S within 5.0Å range:
|
Fluorine binding site 4 out of 6 in 2v0n
Go back to
Fluorine binding site 4 out
of 6 in the Activated Response Regulator Pled in Complex with C-Digmp and Gtp- Alpha-S
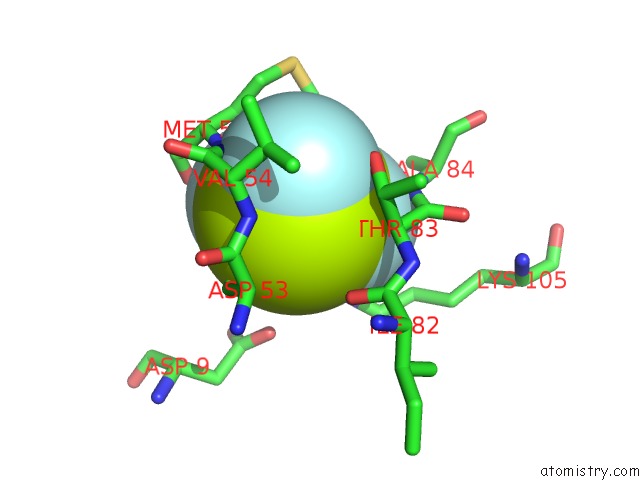
Mono view
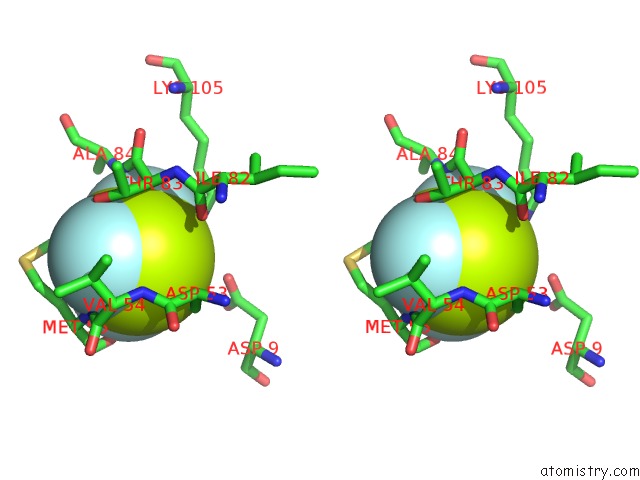
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 4 of Activated Response Regulator Pled in Complex with C-Digmp and Gtp- Alpha-S within 5.0Å range:
|
Fluorine binding site 5 out of 6 in 2v0n
Go back to
Fluorine binding site 5 out
of 6 in the Activated Response Regulator Pled in Complex with C-Digmp and Gtp- Alpha-S
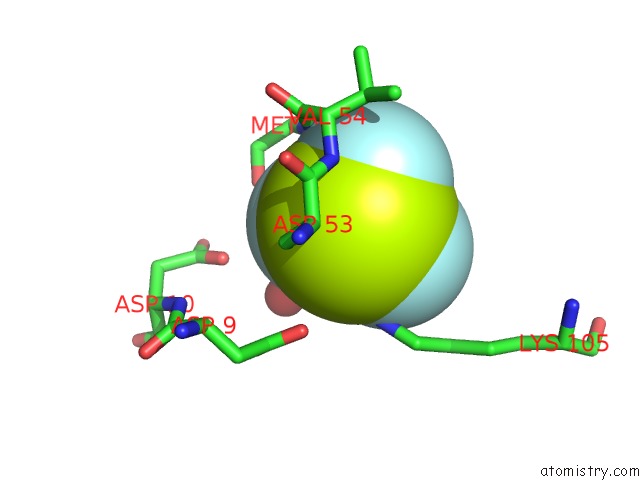
Mono view
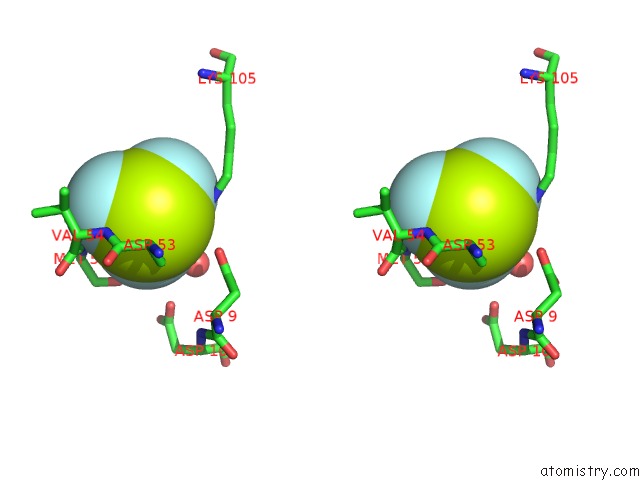
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 5 of Activated Response Regulator Pled in Complex with C-Digmp and Gtp- Alpha-S within 5.0Å range:
|
Fluorine binding site 6 out of 6 in 2v0n
Go back to
Fluorine binding site 6 out
of 6 in the Activated Response Regulator Pled in Complex with C-Digmp and Gtp- Alpha-S
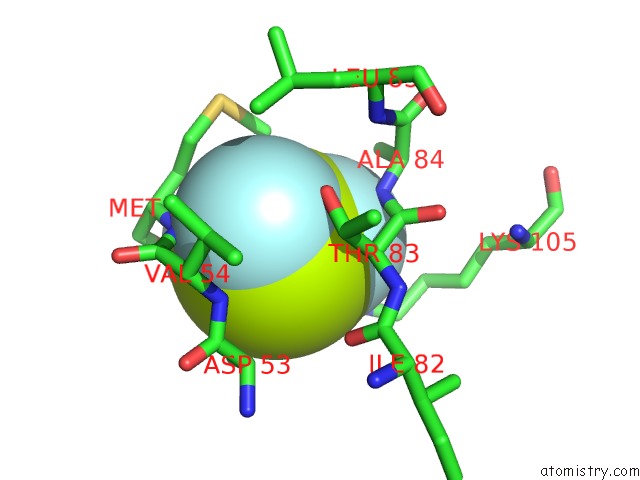
Mono view
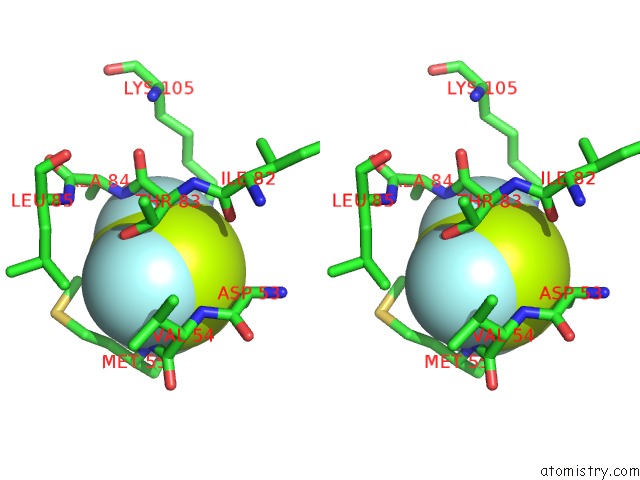
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 6 of Activated Response Regulator Pled in Complex with C-Digmp and Gtp- Alpha-S within 5.0Å range:
|
Reference:
P.Wassmann,
C.Chan,
R.Paul,
A.Beck,
H.Heerklotz,
U.Jenal,
T.Schirmer.
Structure of BEF3--Modified Response Regulator Pled: Implications For Diguanylate Cyclase Activation, Catalysis, and Feedback Inhibition Structure V. 15 915 2007.
ISSN: ISSN 0969-2126
PubMed: 17697997
DOI: 10.1016/J.STR.2007.06.016
Page generated: Wed Jul 31 16:02:08 2024
ISSN: ISSN 0969-2126
PubMed: 17697997
DOI: 10.1016/J.STR.2007.06.016
Last articles
Cl in 5RH1Cl in 5RFU
Cl in 5RFP
Cl in 5RFH
Cl in 5RET
Cl in 5RFF
Cl in 5RB7
Cl in 5RB6
Cl in 5REI
Cl in 5RB4