Fluorine »
PDB 3fls-3g70 »
3g3d »
Fluorine in PDB 3g3d: Crystal Structure of Human Orotidine 5'-Monophosphate Decarboxylase Covalently Modified By 5-Fluoro-6-Azido-Ump
Enzymatic activity of Crystal Structure of Human Orotidine 5'-Monophosphate Decarboxylase Covalently Modified By 5-Fluoro-6-Azido-Ump
All present enzymatic activity of Crystal Structure of Human Orotidine 5'-Monophosphate Decarboxylase Covalently Modified By 5-Fluoro-6-Azido-Ump:
4.1.1.23;
4.1.1.23;
Protein crystallography data
The structure of Crystal Structure of Human Orotidine 5'-Monophosphate Decarboxylase Covalently Modified By 5-Fluoro-6-Azido-Ump, PDB code: 3g3d
was solved by
Y.Liu,
H.L.Tang,
A.Bello,
E.Poduch,
L.Kotra,
E.Pai,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 28.72 / 1.70 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 69.682, 61.850, 70.320, 90.00, 112.73, 90.00 |
| R / Rfree (%) | 17.5 / 21 |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Crystal Structure of Human Orotidine 5'-Monophosphate Decarboxylase Covalently Modified By 5-Fluoro-6-Azido-Ump
(pdb code 3g3d). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 2 binding sites of Fluorine where determined in the Crystal Structure of Human Orotidine 5'-Monophosphate Decarboxylase Covalently Modified By 5-Fluoro-6-Azido-Ump, PDB code: 3g3d:
Jump to Fluorine binding site number: 1; 2;
In total 2 binding sites of Fluorine where determined in the Crystal Structure of Human Orotidine 5'-Monophosphate Decarboxylase Covalently Modified By 5-Fluoro-6-Azido-Ump, PDB code: 3g3d:
Jump to Fluorine binding site number: 1; 2;
Fluorine binding site 1 out of 2 in 3g3d
Go back to
Fluorine binding site 1 out
of 2 in the Crystal Structure of Human Orotidine 5'-Monophosphate Decarboxylase Covalently Modified By 5-Fluoro-6-Azido-Ump
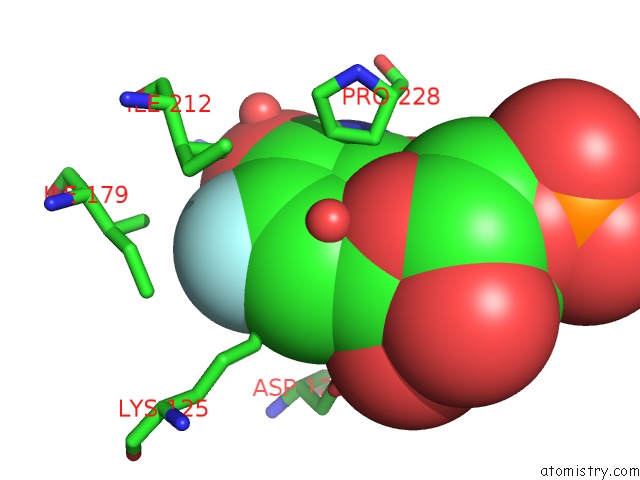
Mono view
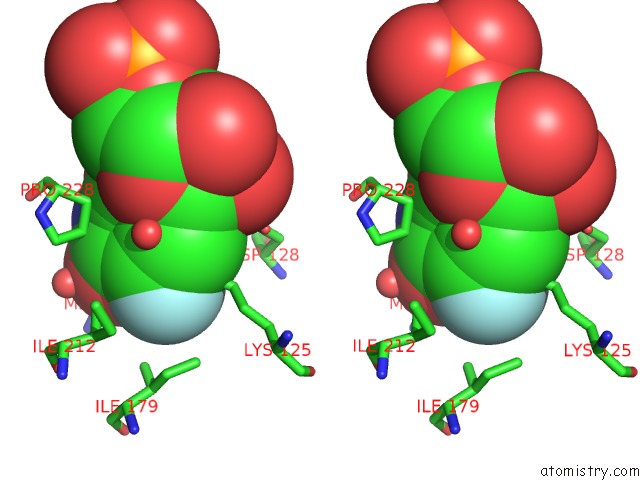
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Crystal Structure of Human Orotidine 5'-Monophosphate Decarboxylase Covalently Modified By 5-Fluoro-6-Azido-Ump within 5.0Å range:
|
Fluorine binding site 2 out of 2 in 3g3d
Go back to
Fluorine binding site 2 out
of 2 in the Crystal Structure of Human Orotidine 5'-Monophosphate Decarboxylase Covalently Modified By 5-Fluoro-6-Azido-Ump
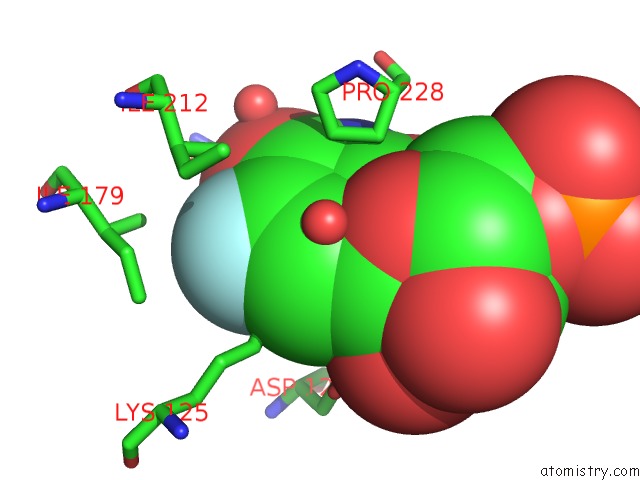
Mono view
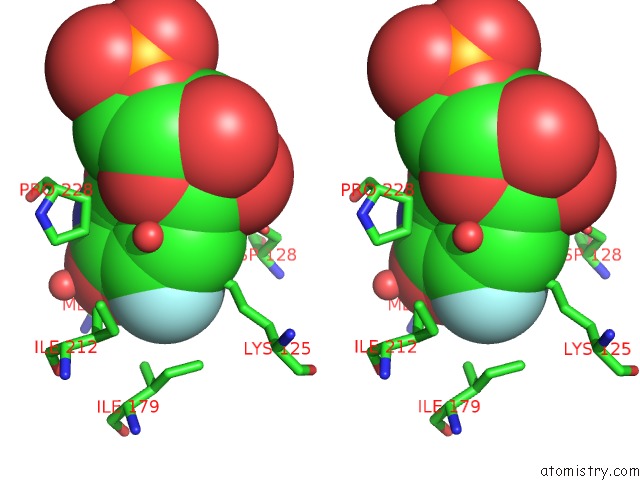
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Crystal Structure of Human Orotidine 5'-Monophosphate Decarboxylase Covalently Modified By 5-Fluoro-6-Azido-Ump within 5.0Å range:
|
Reference:
A.M.Bello,
D.Konforte,
E.Poduch,
C.Furlonger,
L.Wei,
Y.Liu,
M.Lewis,
E.F.Pai,
C.J.Paige,
L.P.Kotra.
Structure-Activity Relationships of Orotidine-5'-Monophosphate Decarboxylase Inhibitors As Anticancer Agents. J.Med.Chem. V. 52 1648 2009.
ISSN: ISSN 0022-2623
PubMed: 19260677
DOI: 10.1021/JM801224T
Page generated: Wed Jul 31 18:44:09 2024
ISSN: ISSN 0022-2623
PubMed: 19260677
DOI: 10.1021/JM801224T
Last articles
Ca in 3C63Ca in 3C62
Ca in 3C5I
Ca in 3C1V
Ca in 3C52
Ca in 3C22
Ca in 3C3Y
Ca in 3C25
Ca in 3C1Q
Ca in 3C1F