Fluorine »
PDB 3ohh-3oyj »
3omq »
Fluorine in PDB 3omq: Fragment-Based Design of Novel Estrogen Receptor Ligands
Protein crystallography data
The structure of Fragment-Based Design of Novel Estrogen Receptor Ligands, PDB code: 3omq
was solved by
S.Moecklinghoff,
W.A.Van Otterlo,
R.Rose,
S.Fuchs,
M.Dominguez Seoane,
H.Waldmann,
C.Ottmann,
L.Brunsveld,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 19.55 / 1.97 |
| Space group | P 31 |
| Cell size a, b, c (Å), α, β, γ (°) | 70.590, 70.590, 110.090, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 20.5 / 26 |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Fragment-Based Design of Novel Estrogen Receptor Ligands
(pdb code 3omq). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 6 binding sites of Fluorine where determined in the Fragment-Based Design of Novel Estrogen Receptor Ligands, PDB code: 3omq:
Jump to Fluorine binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Fluorine where determined in the Fragment-Based Design of Novel Estrogen Receptor Ligands, PDB code: 3omq:
Jump to Fluorine binding site number: 1; 2; 3; 4; 5; 6;
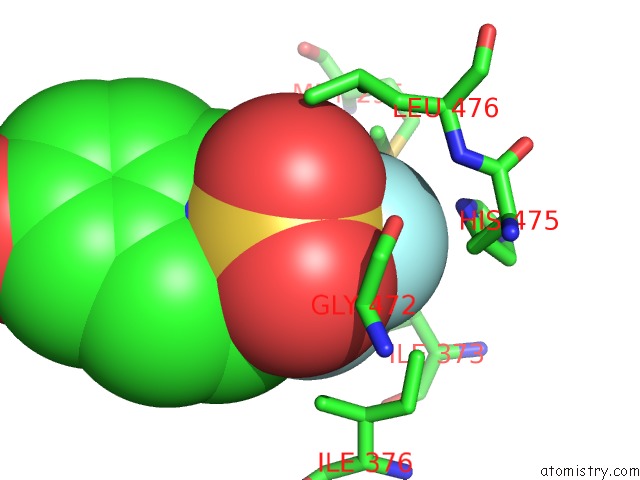
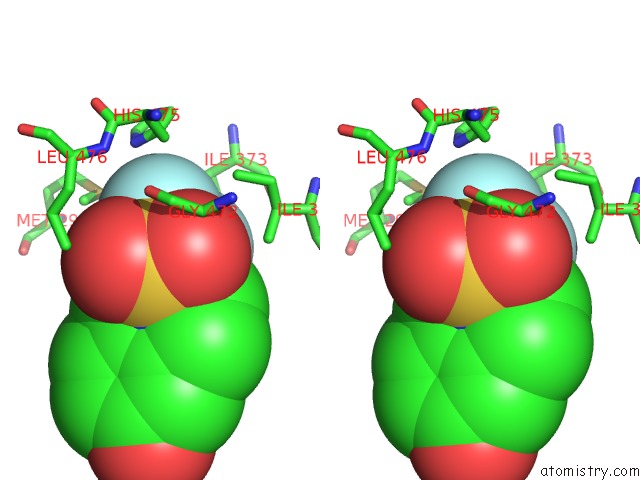
Fluorine binding site 1 out of 6 in 3omq
Go back to
Fluorine binding site 1 out
of 6 in the Fragment-Based Design of Novel Estrogen Receptor Ligands
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Fragment-Based Design of Novel Estrogen Receptor Ligands within 5.0Å range:
|
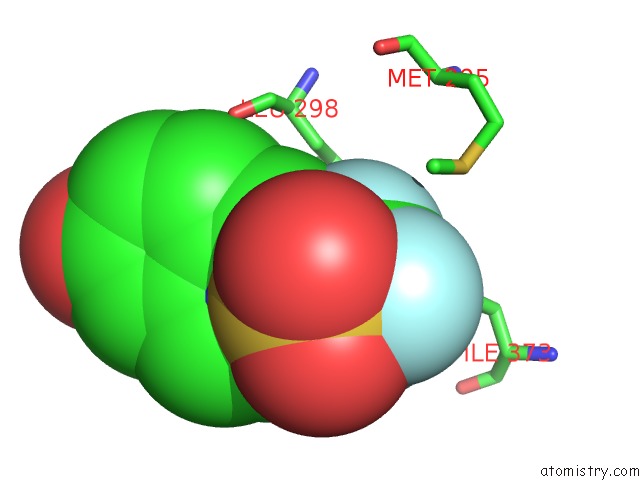
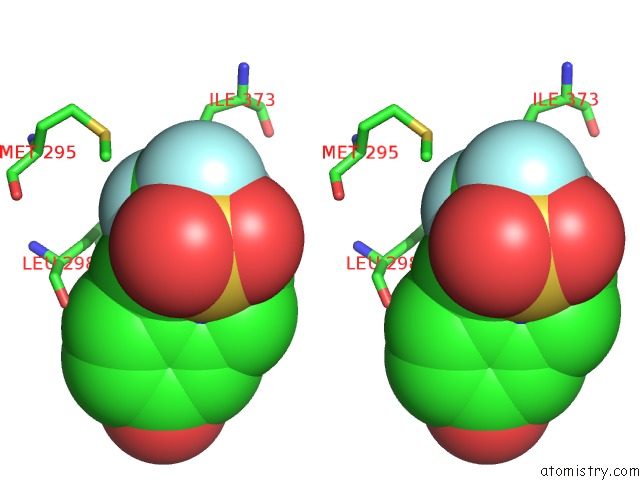
Fluorine binding site 2 out of 6 in 3omq
Go back to
Fluorine binding site 2 out
of 6 in the Fragment-Based Design of Novel Estrogen Receptor Ligands
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Fragment-Based Design of Novel Estrogen Receptor Ligands within 5.0Å range:
|
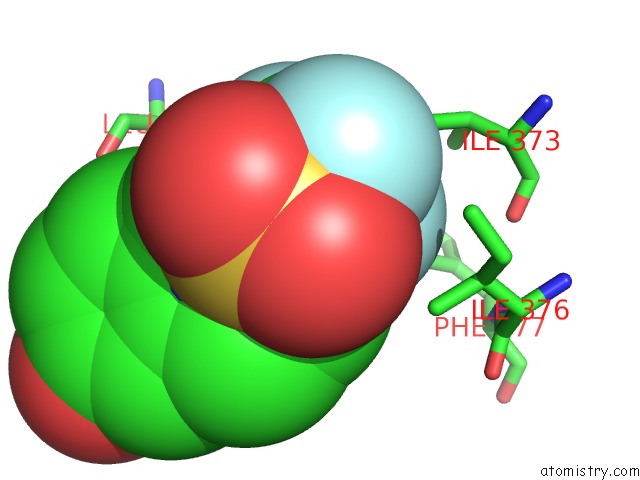
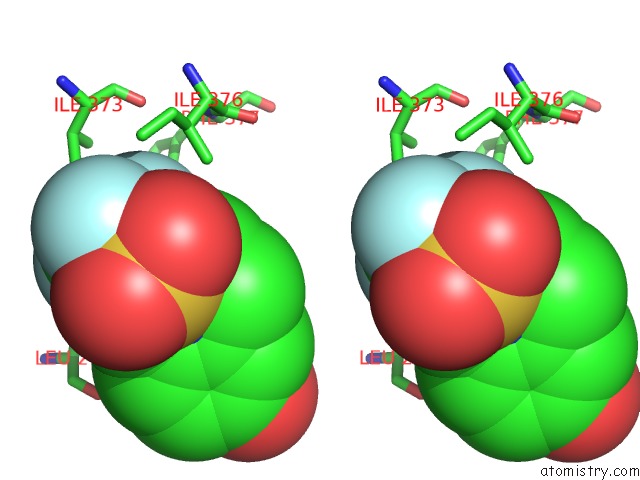
Fluorine binding site 3 out of 6 in 3omq
Go back to
Fluorine binding site 3 out
of 6 in the Fragment-Based Design of Novel Estrogen Receptor Ligands
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Fragment-Based Design of Novel Estrogen Receptor Ligands within 5.0Å range:
|
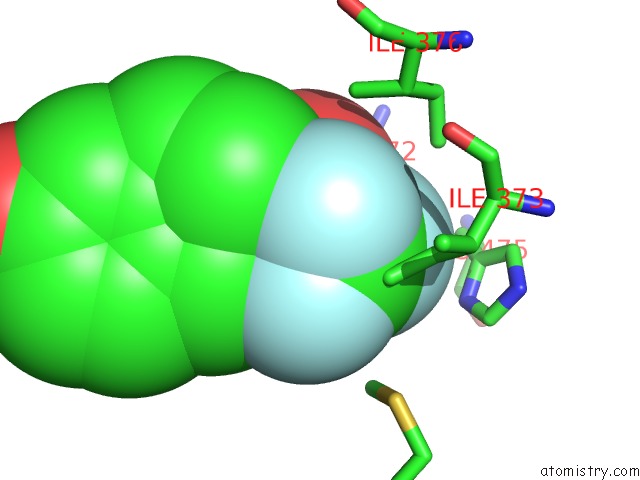
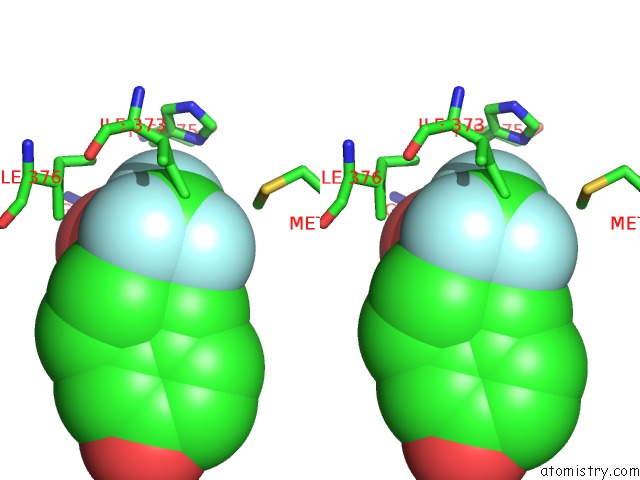
Fluorine binding site 4 out of 6 in 3omq
Go back to
Fluorine binding site 4 out
of 6 in the Fragment-Based Design of Novel Estrogen Receptor Ligands
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 4 of Fragment-Based Design of Novel Estrogen Receptor Ligands within 5.0Å range:
|
Fluorine binding site 5 out of 6 in 3omq
Go back to
Fluorine binding site 5 out
of 6 in the Fragment-Based Design of Novel Estrogen Receptor Ligands
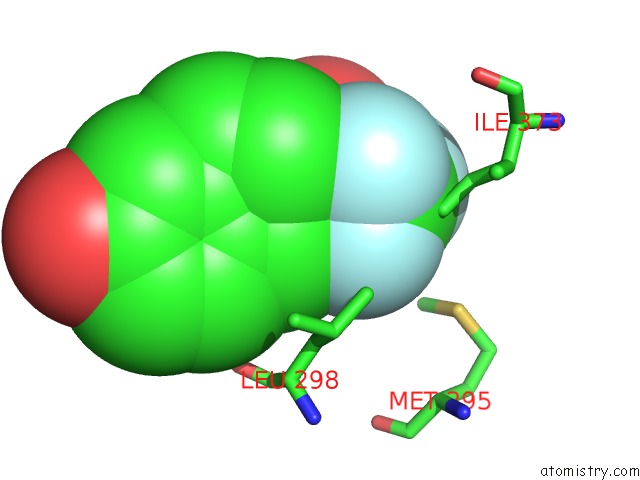
Mono view
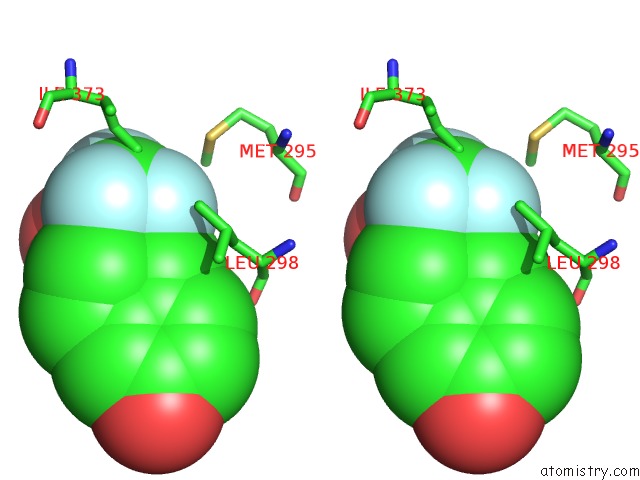
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 5 of Fragment-Based Design of Novel Estrogen Receptor Ligands within 5.0Å range:
|
Fluorine binding site 6 out of 6 in 3omq
Go back to
Fluorine binding site 6 out
of 6 in the Fragment-Based Design of Novel Estrogen Receptor Ligands
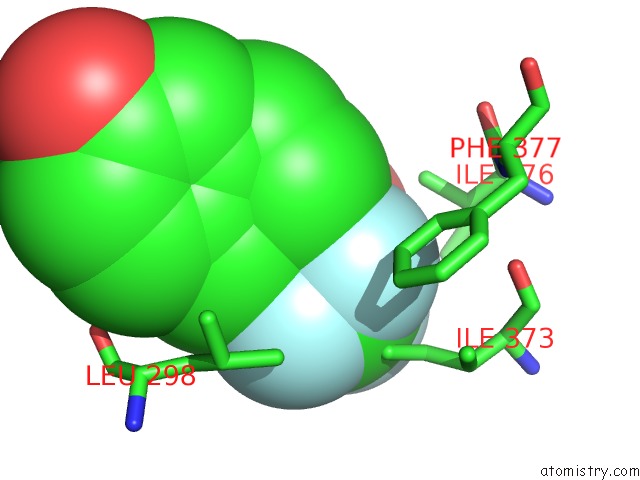
Mono view
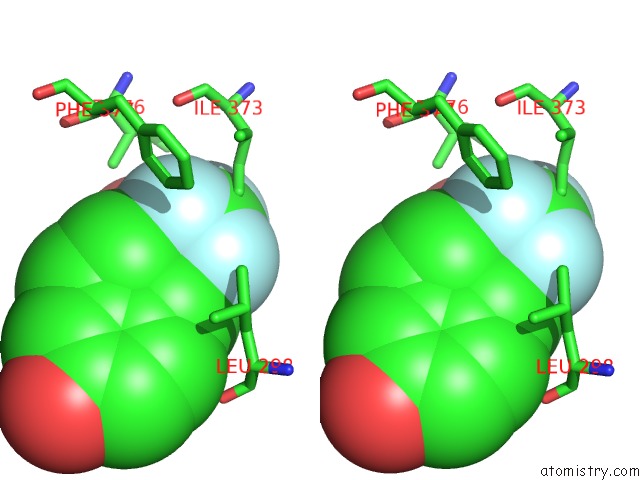
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 6 of Fragment-Based Design of Novel Estrogen Receptor Ligands within 5.0Å range:
|
Reference:
W.A.Van Otterlo,
R.Rose,
S.Fuchs,
T.J.Zimmermann,
M.Dominguez Seoane,
H.Waldmann,
C.Ottmann,
L.Brunsveld.
Design and Evaluation of Fragment-Like Estrogen Receptor Tetrahydroisoquinoline Ligands From A Scaffold-Detection Approach. J.Med.Chem. V. 54 2005 2011.
ISSN: ISSN 0022-2623
PubMed: 21381753
DOI: 10.1021/JM1011116
Page generated: Wed Jul 31 21:19:18 2024
ISSN: ISSN 0022-2623
PubMed: 21381753
DOI: 10.1021/JM1011116
Last articles
Zn in 9J0NZn in 9J0O
Zn in 9J0P
Zn in 9FJX
Zn in 9EKB
Zn in 9C0F
Zn in 9CAH
Zn in 9CH0
Zn in 9CH3
Zn in 9CH1