Fluorine »
PDB 3ptm-3qri »
3qiz »
Fluorine in PDB 3qiz: Crystal Structure of Bont/A Lc Complexed with Hydroxamate-Based Inhibitor Pt-2
Enzymatic activity of Crystal Structure of Bont/A Lc Complexed with Hydroxamate-Based Inhibitor Pt-2
All present enzymatic activity of Crystal Structure of Bont/A Lc Complexed with Hydroxamate-Based Inhibitor Pt-2:
3.4.24.69;
3.4.24.69;
Protein crystallography data
The structure of Crystal Structure of Bont/A Lc Complexed with Hydroxamate-Based Inhibitor Pt-2, PDB code: 3qiz
was solved by
A.A.Thompson,
G.W.Han,
R.C.Stevens,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 37.13 / 2.00 |
| Space group | P 21 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 59.230, 190.654, 42.430, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 18.8 / 22.3 |
Other elements in 3qiz:
The structure of Crystal Structure of Bont/A Lc Complexed with Hydroxamate-Based Inhibitor Pt-2 also contains other interesting chemical elements:
| Zinc | (Zn) | 1 atom |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Crystal Structure of Bont/A Lc Complexed with Hydroxamate-Based Inhibitor Pt-2
(pdb code 3qiz). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 2 binding sites of Fluorine where determined in the Crystal Structure of Bont/A Lc Complexed with Hydroxamate-Based Inhibitor Pt-2, PDB code: 3qiz:
Jump to Fluorine binding site number: 1; 2;
In total 2 binding sites of Fluorine where determined in the Crystal Structure of Bont/A Lc Complexed with Hydroxamate-Based Inhibitor Pt-2, PDB code: 3qiz:
Jump to Fluorine binding site number: 1; 2;
Fluorine binding site 1 out of 2 in 3qiz
Go back to
Fluorine binding site 1 out
of 2 in the Crystal Structure of Bont/A Lc Complexed with Hydroxamate-Based Inhibitor Pt-2
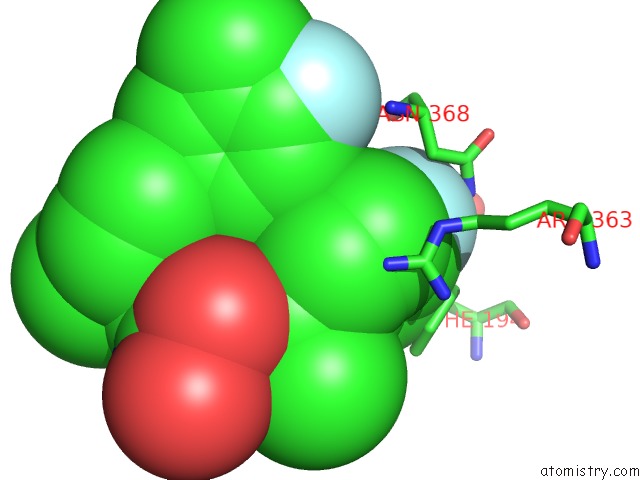
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Crystal Structure of Bont/A Lc Complexed with Hydroxamate-Based Inhibitor Pt-2 within 5.0Å range:
|
Fluorine binding site 2 out of 2 in 3qiz
Go back to
Fluorine binding site 2 out
of 2 in the Crystal Structure of Bont/A Lc Complexed with Hydroxamate-Based Inhibitor Pt-2
Mono view
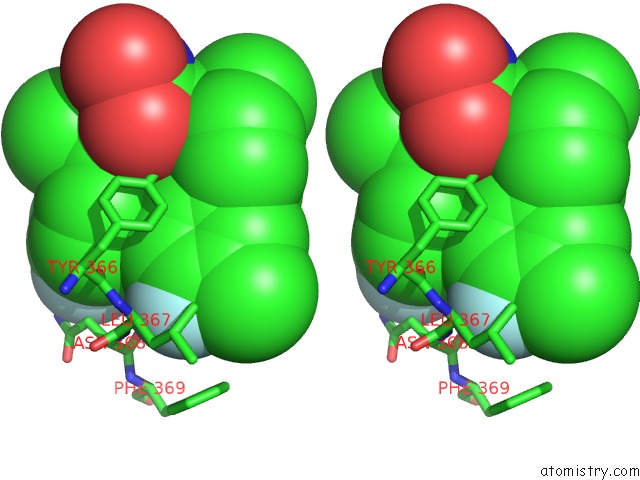
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Crystal Structure of Bont/A Lc Complexed with Hydroxamate-Based Inhibitor Pt-2 within 5.0Å range:
|
Reference:
A.A.Thompson,
G.S.Jiao,
S.Kim,
A.Thai,
L.Cregar-Hernandez,
S.A.Margosiak,
A.T.Johnson,
G.W.Han,
S.O'malley,
R.C.Stevens.
Structural Characterization of Three Novel Hydroxamate-Based Zinc Chelating Inhibitors of the Clostridium Botulinum Serotype A Neurotoxin Light Chain Metalloprotease Reveals A Compact Binding Site Resulting From 60/70 Loop Flexibility. Biochemistry V. 50 4019 2011.
ISSN: ISSN 0006-2960
PubMed: 21434688
DOI: 10.1021/BI2001483
Page generated: Wed Jul 31 22:03:39 2024
ISSN: ISSN 0006-2960
PubMed: 21434688
DOI: 10.1021/BI2001483
Last articles
Ca in 5TLICa in 5TLN
Ca in 5TH6
Ca in 5TL8
Ca in 5TIW
Ca in 5TJ6
Ca in 5TH9
Ca in 5TIS
Ca in 5TI8
Ca in 5THG