Fluorine »
PDB 4bkz-4c62 »
4bs5 »
Fluorine in PDB 4bs5: Mouse Cathepsin S with Covalent Ligand
Enzymatic activity of Mouse Cathepsin S with Covalent Ligand
All present enzymatic activity of Mouse Cathepsin S with Covalent Ligand:
3.4.22.27;
3.4.22.27;
Protein crystallography data
The structure of Mouse Cathepsin S with Covalent Ligand, PDB code: 4bs5
was solved by
D.W.Banner,
J.Benz,
B.Gsell,
M.Stihle,
A.Ruf,
W.Haap,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 35.69 / 1.25 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 74.640, 66.790, 54.320, 90.00, 114.06, 90.00 |
| R / Rfree (%) | 19.71 / 22.694 |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Mouse Cathepsin S with Covalent Ligand
(pdb code 4bs5). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 3 binding sites of Fluorine where determined in the Mouse Cathepsin S with Covalent Ligand, PDB code: 4bs5:
Jump to Fluorine binding site number: 1; 2; 3;
In total 3 binding sites of Fluorine where determined in the Mouse Cathepsin S with Covalent Ligand, PDB code: 4bs5:
Jump to Fluorine binding site number: 1; 2; 3;
Fluorine binding site 1 out of 3 in 4bs5
Go back to
Fluorine binding site 1 out
of 3 in the Mouse Cathepsin S with Covalent Ligand
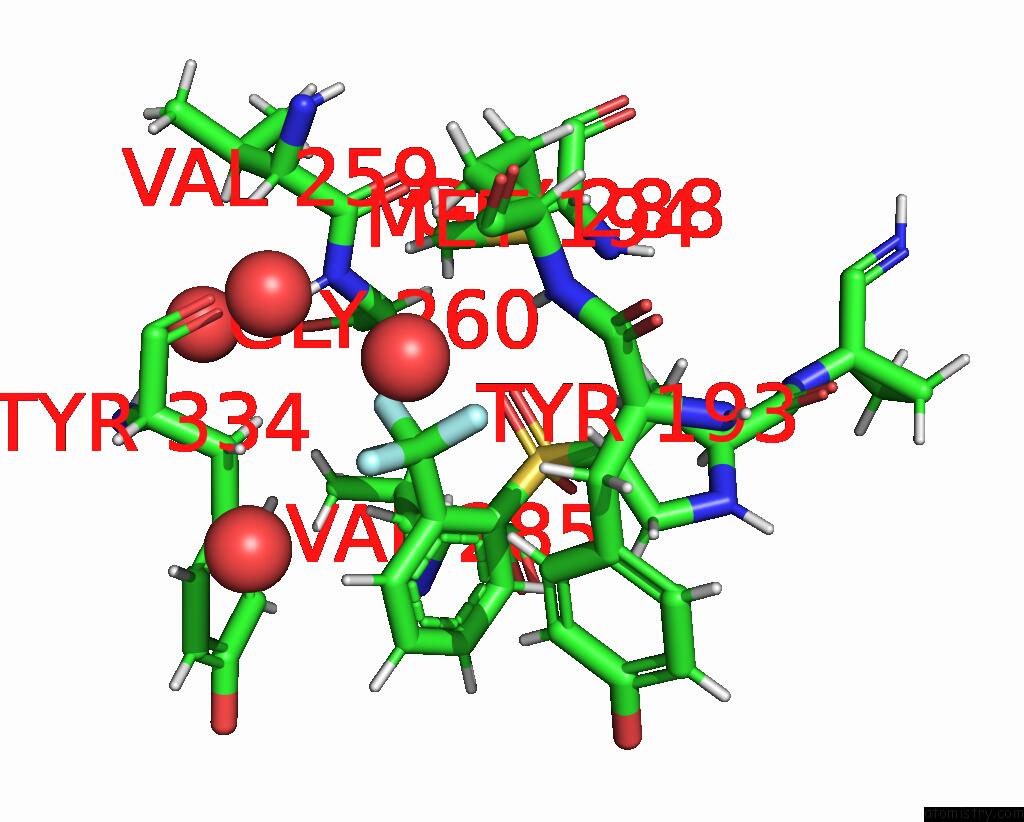
Mono view
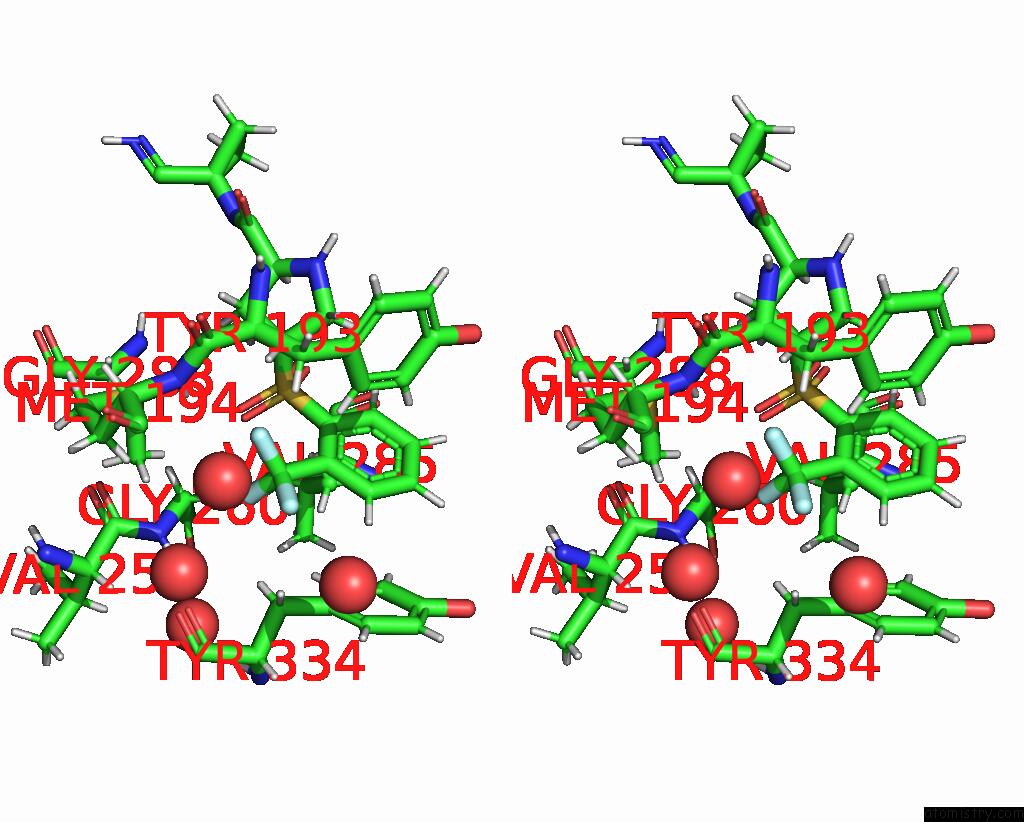
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Mouse Cathepsin S with Covalent Ligand within 5.0Å range:
|
Fluorine binding site 2 out of 3 in 4bs5
Go back to
Fluorine binding site 2 out
of 3 in the Mouse Cathepsin S with Covalent Ligand
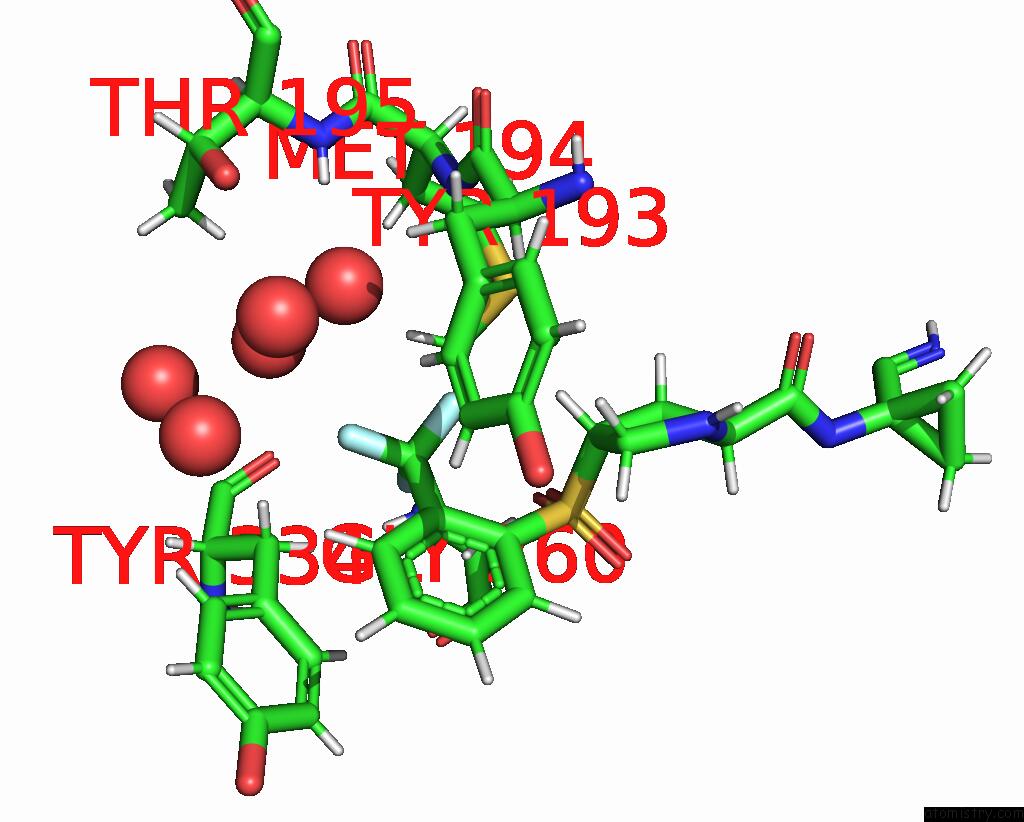
Mono view
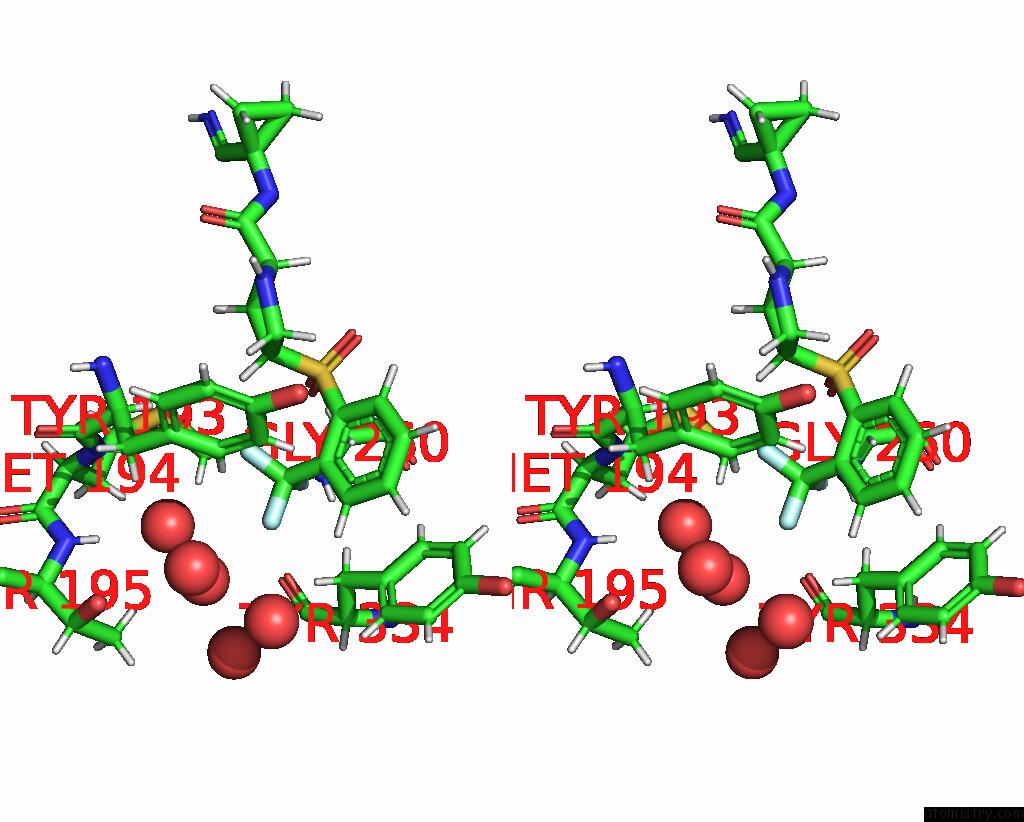
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Mouse Cathepsin S with Covalent Ligand within 5.0Å range:
|
Fluorine binding site 3 out of 3 in 4bs5
Go back to
Fluorine binding site 3 out
of 3 in the Mouse Cathepsin S with Covalent Ligand
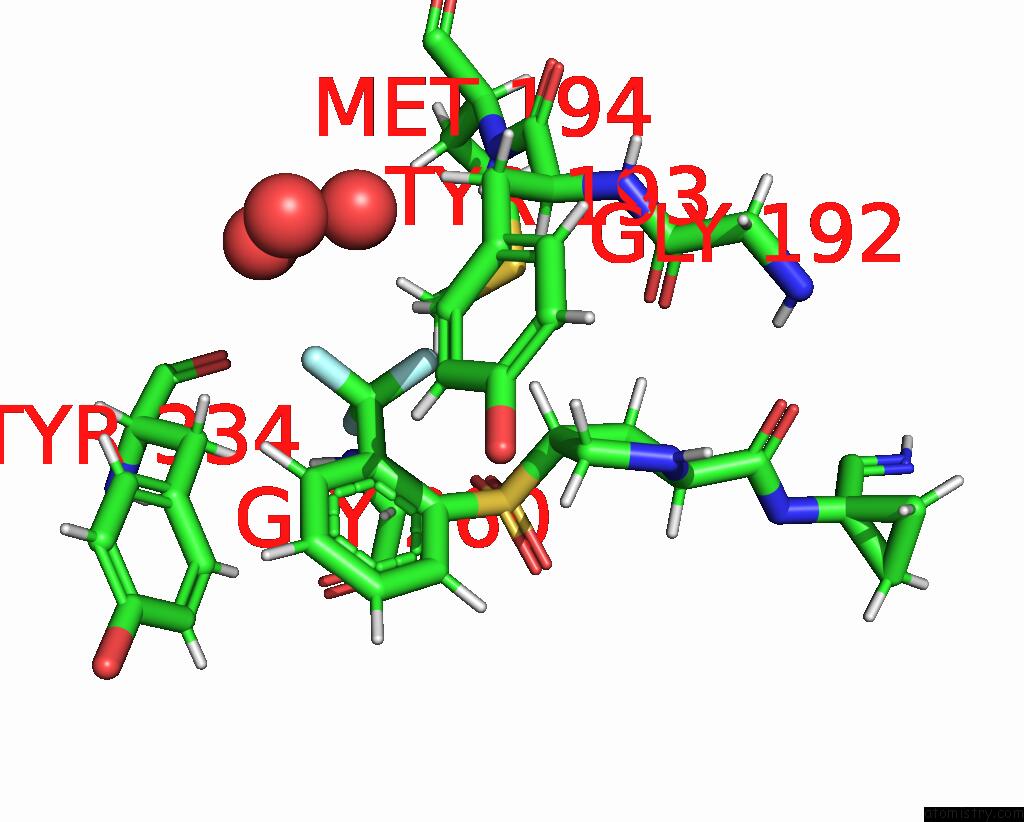
Mono view
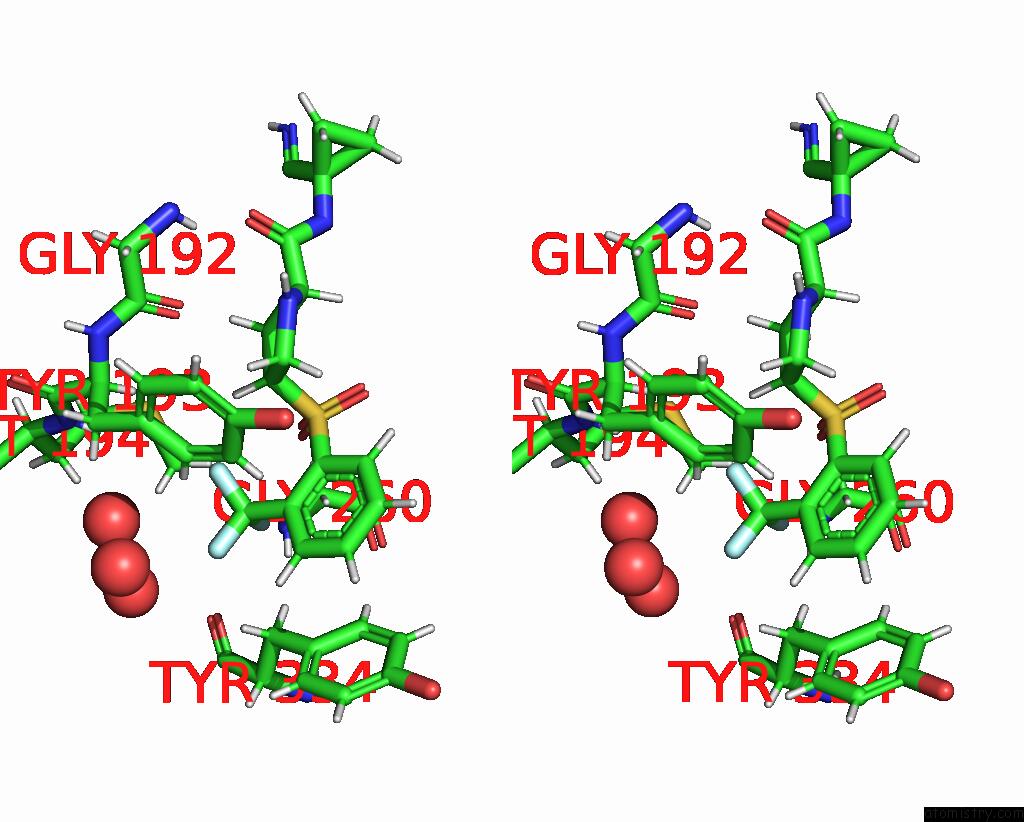
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Mouse Cathepsin S with Covalent Ligand within 5.0Å range:
|
Reference:
H.Hilpert,
H.Mauser,
R.Humm,
L.Anselm,
H.Kuehne,
G.Hartmann,
S.Gruener,
D.W.Banner,
J.Benz,
B.Gsell,
A.Kuglstatter,
M.Stihle,
R.Thoma,
R.Alvarez-Sanchez,
H.Iding,
B.Wirz,
W.Haap.
Identification of Potent and Selective Cathepsin S (Cats) Inhibitors Containing Different Central Cyclic Scaffolds. J.Med.Chem. V. 56 9789 2013.
ISSN: ISSN 0022-2623
PubMed: 24224654
DOI: 10.1021/JM401528K
Page generated: Mon Jul 14 20:41:59 2025
ISSN: ISSN 0022-2623
PubMed: 24224654
DOI: 10.1021/JM401528K
Last articles
F in 4XDKF in 4XQ0
F in 4XPZ
F in 4XNV
F in 4XMO
F in 4XPG
F in 4XKX
F in 4XM7
F in 4XM6
F in 4XJU