Fluorine »
PDB 5fd2-5g3j »
5fr0 »
Fluorine in PDB 5fr0: The Details of Glycolipid Glycan Hydrolysis By the Structural Analysis of A Family 123 Glycoside Hydrolase From Clostridium Perfringens
Protein crystallography data
The structure of The Details of Glycolipid Glycan Hydrolysis By the Structural Analysis of A Family 123 Glycoside Hydrolase From Clostridium Perfringens, PDB code: 5fr0
was solved by
I.Noach,
B.Pluvinage,
C.Laurie,
K.T.Abe,
M.Alteen,
D.J.Vocadlo,
A.B.Boraston,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 95.31 / 1.75 |
| Space group | P 32 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 99.530, 99.530, 95.310, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 18.074 / 22.099 |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the The Details of Glycolipid Glycan Hydrolysis By the Structural Analysis of A Family 123 Glycoside Hydrolase From Clostridium Perfringens
(pdb code 5fr0). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 2 binding sites of Fluorine where determined in the The Details of Glycolipid Glycan Hydrolysis By the Structural Analysis of A Family 123 Glycoside Hydrolase From Clostridium Perfringens, PDB code: 5fr0:
Jump to Fluorine binding site number: 1; 2;
In total 2 binding sites of Fluorine where determined in the The Details of Glycolipid Glycan Hydrolysis By the Structural Analysis of A Family 123 Glycoside Hydrolase From Clostridium Perfringens, PDB code: 5fr0:
Jump to Fluorine binding site number: 1; 2;
Fluorine binding site 1 out of 2 in 5fr0
Go back to
Fluorine binding site 1 out
of 2 in the The Details of Glycolipid Glycan Hydrolysis By the Structural Analysis of A Family 123 Glycoside Hydrolase From Clostridium Perfringens
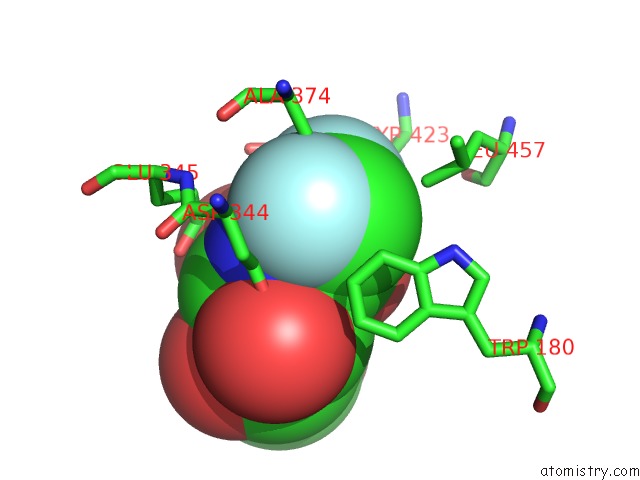
Mono view
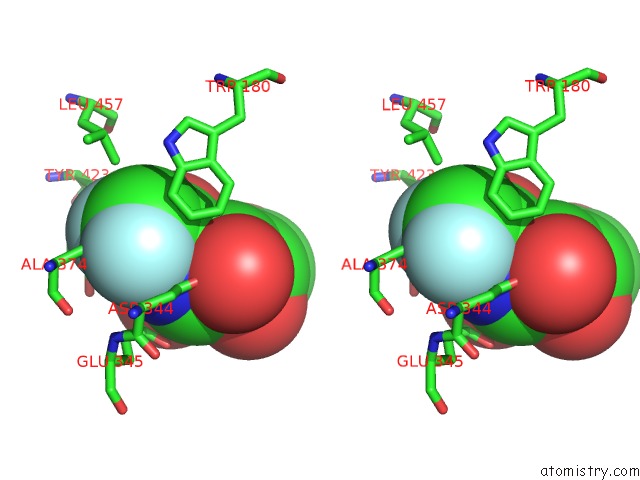
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of The Details of Glycolipid Glycan Hydrolysis By the Structural Analysis of A Family 123 Glycoside Hydrolase From Clostridium Perfringens within 5.0Å range:
|
Fluorine binding site 2 out of 2 in 5fr0
Go back to
Fluorine binding site 2 out
of 2 in the The Details of Glycolipid Glycan Hydrolysis By the Structural Analysis of A Family 123 Glycoside Hydrolase From Clostridium Perfringens
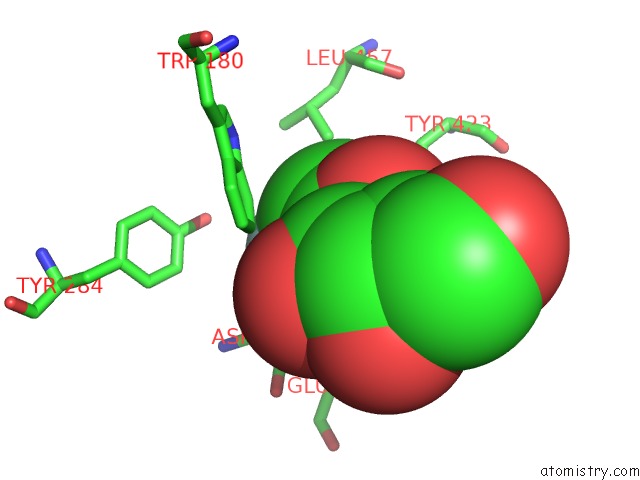
Mono view
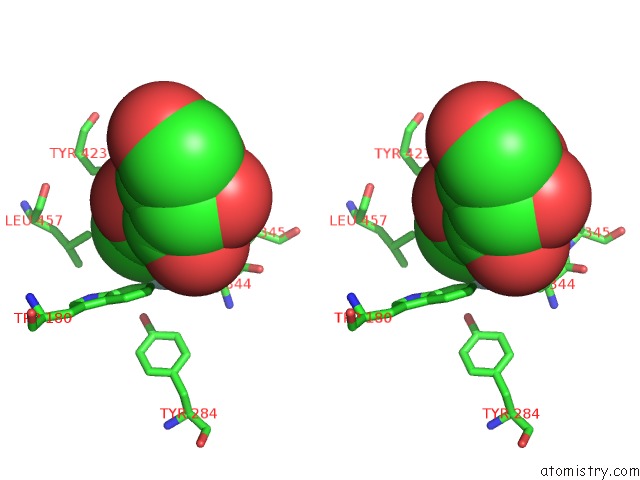
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of The Details of Glycolipid Glycan Hydrolysis By the Structural Analysis of A Family 123 Glycoside Hydrolase From Clostridium Perfringens within 5.0Å range:
|
Reference:
I.Noach,
B.Pluvinage,
C.Laurie,
K.T.Abe,
M.Alteen,
D.J.Vocadlo,
A.B.Boraston.
The Details of Glycolipid Glycan Hydrolysis By the Structural Analysis of A Family 123 Glycoside Hydrolase From Clostridium Perfringens J.Mol.Biol. V. 428 3253 2016.
ISSN: ISSN 0022-2836
PubMed: 27038508
DOI: 10.1016/J.JMB.2016.03.020
Page generated: Tue Jul 15 03:33:40 2025
ISSN: ISSN 0022-2836
PubMed: 27038508
DOI: 10.1016/J.JMB.2016.03.020
Last articles
F in 5TCIF in 5TBO
F in 5TCO
F in 5TCJ
F in 5T2P
F in 5SWM
F in 5TBM
F in 5TBE
F in 5TBB
F in 5TA8