Fluorine »
PDB 5hu9-5ifd »
5i9m »
Fluorine in PDB 5i9m: Crystal Structure of B. Pseudomallei Fabi in Complex with Nad and PT408
Enzymatic activity of Crystal Structure of B. Pseudomallei Fabi in Complex with Nad and PT408
All present enzymatic activity of Crystal Structure of B. Pseudomallei Fabi in Complex with Nad and PT408:
1.3.1.9;
1.3.1.9;
Protein crystallography data
The structure of Crystal Structure of B. Pseudomallei Fabi in Complex with Nad and PT408, PDB code: 5i9m
was solved by
M.W.Hirschbeck,
S.Eltschkner,
P.J.Tonge,
C.Kisker,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 47.21 / 2.25 |
| Space group | I 2 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 69.029, 111.217, 260.695, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 25.1 / 28.1 |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Crystal Structure of B. Pseudomallei Fabi in Complex with Nad and PT408
(pdb code 5i9m). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 3 binding sites of Fluorine where determined in the Crystal Structure of B. Pseudomallei Fabi in Complex with Nad and PT408, PDB code: 5i9m:
Jump to Fluorine binding site number: 1; 2; 3;
In total 3 binding sites of Fluorine where determined in the Crystal Structure of B. Pseudomallei Fabi in Complex with Nad and PT408, PDB code: 5i9m:
Jump to Fluorine binding site number: 1; 2; 3;
Fluorine binding site 1 out of 3 in 5i9m
Go back to
Fluorine binding site 1 out
of 3 in the Crystal Structure of B. Pseudomallei Fabi in Complex with Nad and PT408
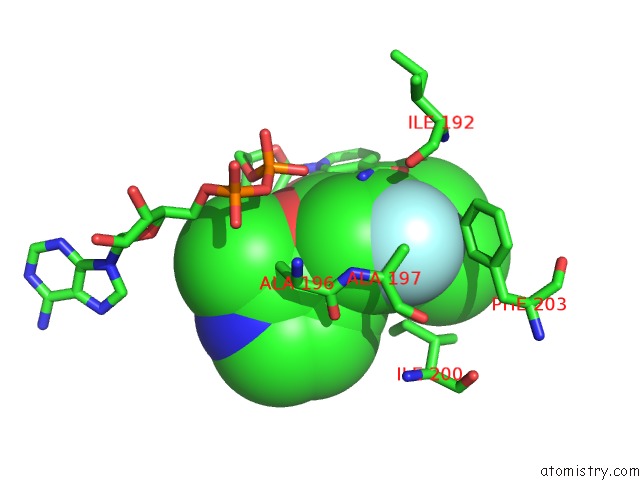
Mono view
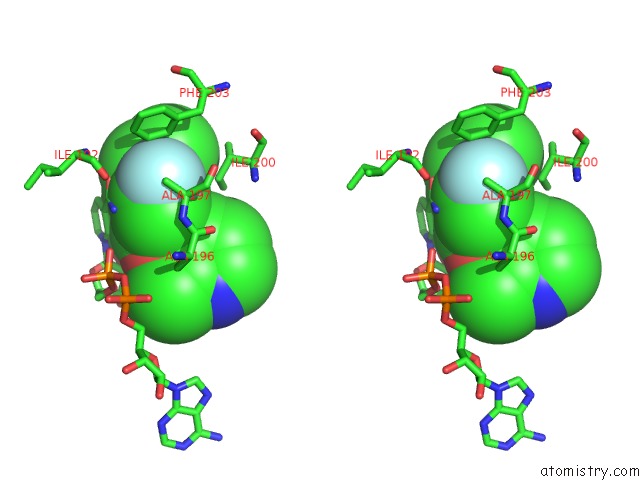
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Crystal Structure of B. Pseudomallei Fabi in Complex with Nad and PT408 within 5.0Å range:
|
Fluorine binding site 2 out of 3 in 5i9m
Go back to
Fluorine binding site 2 out
of 3 in the Crystal Structure of B. Pseudomallei Fabi in Complex with Nad and PT408
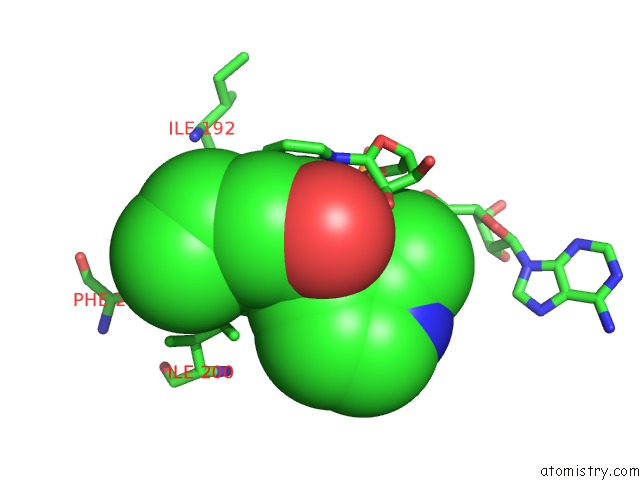
Mono view
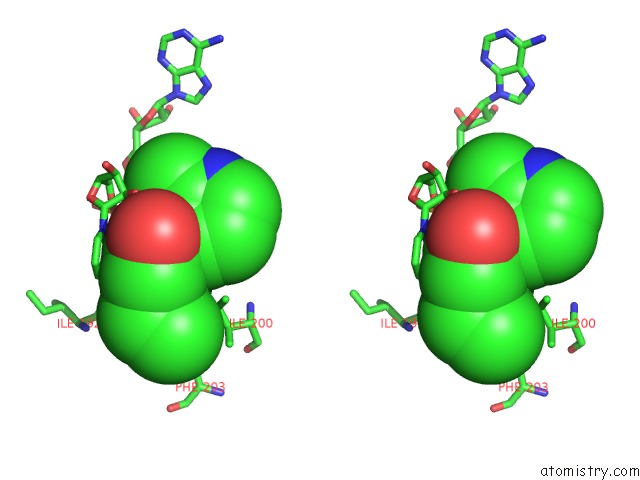
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Crystal Structure of B. Pseudomallei Fabi in Complex with Nad and PT408 within 5.0Å range:
|
Fluorine binding site 3 out of 3 in 5i9m
Go back to
Fluorine binding site 3 out
of 3 in the Crystal Structure of B. Pseudomallei Fabi in Complex with Nad and PT408
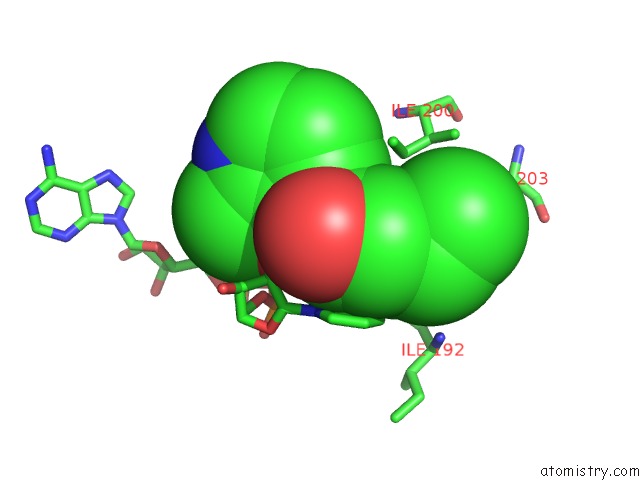
Mono view
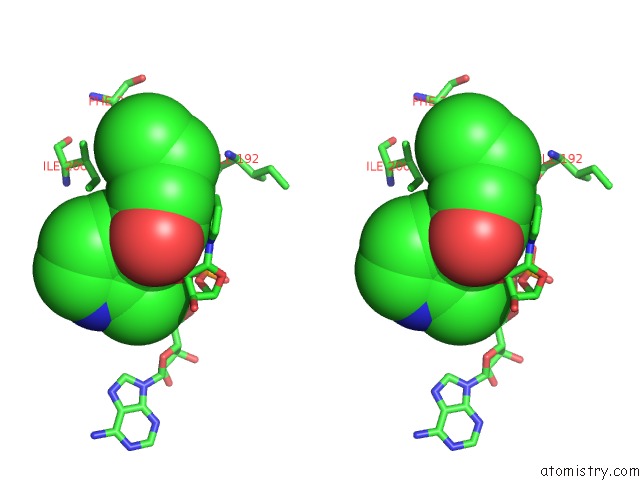
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Crystal Structure of B. Pseudomallei Fabi in Complex with Nad and PT408 within 5.0Å range:
|
Reference:
C.Neckles,
S.Eltschkner,
J.E.Cummings,
M.Hirschbeck,
F.Daryaee,
G.R.Bommineni,
Z.Zhang,
L.Spagnuolo,
W.Yu,
S.Davoodi,
R.A.Slayden,
C.Kisker,
P.J.Tonge.
Rationalizing the Binding Kinetics For the Inhibition of the Burkholderia Pseudomallei FABI1 Enoyl-Acp Reductase. Biochemistry V. 56 1865 2017.
ISSN: ISSN 1520-4995
PubMed: 28225601
DOI: 10.1021/ACS.BIOCHEM.6B01048
Page generated: Tue Jul 15 04:06:37 2025
ISSN: ISSN 1520-4995
PubMed: 28225601
DOI: 10.1021/ACS.BIOCHEM.6B01048
Last articles
F in 6D0JF in 6D0E
F in 6D0N
F in 6D09
F in 6D0B
F in 6D0D
F in 6CZW
F in 6CZI
F in 6CYB
F in 6CZH