Fluorine »
PDB 5jwc-5knj »
5kby »
Fluorine in PDB 5kby: Crystal Structure of Dipeptidyl Peptidase IV in Complex with Syr-472
Enzymatic activity of Crystal Structure of Dipeptidyl Peptidase IV in Complex with Syr-472
All present enzymatic activity of Crystal Structure of Dipeptidyl Peptidase IV in Complex with Syr-472:
3.4.14.5;
3.4.14.5;
Protein crystallography data
The structure of Crystal Structure of Dipeptidyl Peptidase IV in Complex with Syr-472, PDB code: 5kby
was solved by
R.J.Skene,
A.J.Jennings,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 34.57 / 2.24 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 121.573, 122.165, 143.704, 90.00, 114.57, 90.00 |
| R / Rfree (%) | 17.4 / 21.5 |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Crystal Structure of Dipeptidyl Peptidase IV in Complex with Syr-472
(pdb code 5kby). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 4 binding sites of Fluorine where determined in the Crystal Structure of Dipeptidyl Peptidase IV in Complex with Syr-472, PDB code: 5kby:
Jump to Fluorine binding site number: 1; 2; 3; 4;
In total 4 binding sites of Fluorine where determined in the Crystal Structure of Dipeptidyl Peptidase IV in Complex with Syr-472, PDB code: 5kby:
Jump to Fluorine binding site number: 1; 2; 3; 4;
Fluorine binding site 1 out of 4 in 5kby
Go back to
Fluorine binding site 1 out
of 4 in the Crystal Structure of Dipeptidyl Peptidase IV in Complex with Syr-472

Mono view
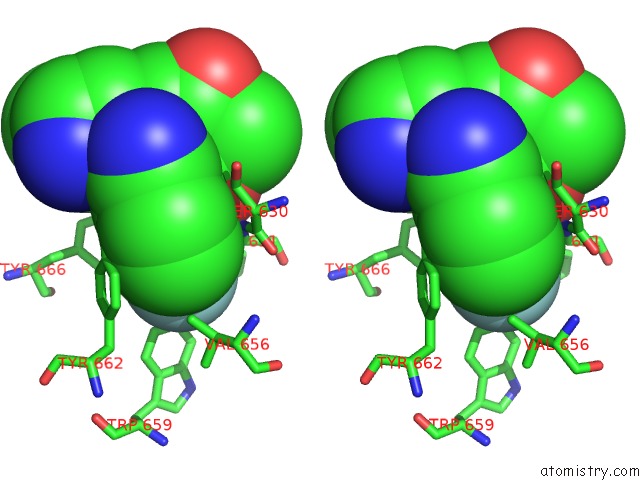
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Crystal Structure of Dipeptidyl Peptidase IV in Complex with Syr-472 within 5.0Å range:
|
Fluorine binding site 2 out of 4 in 5kby
Go back to
Fluorine binding site 2 out
of 4 in the Crystal Structure of Dipeptidyl Peptidase IV in Complex with Syr-472
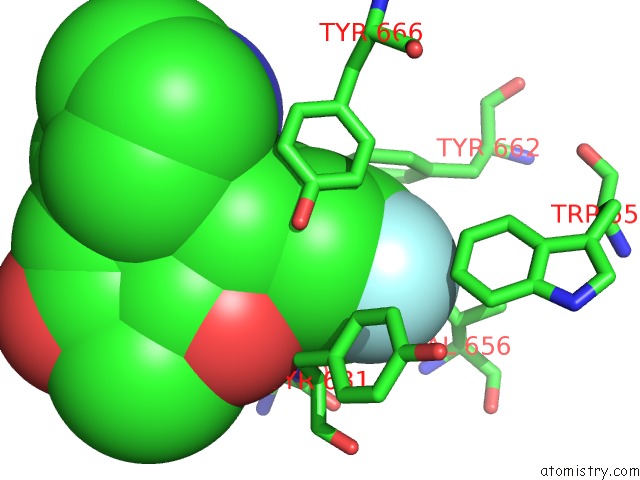
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Crystal Structure of Dipeptidyl Peptidase IV in Complex with Syr-472 within 5.0Å range:
|
Fluorine binding site 3 out of 4 in 5kby
Go back to
Fluorine binding site 3 out
of 4 in the Crystal Structure of Dipeptidyl Peptidase IV in Complex with Syr-472
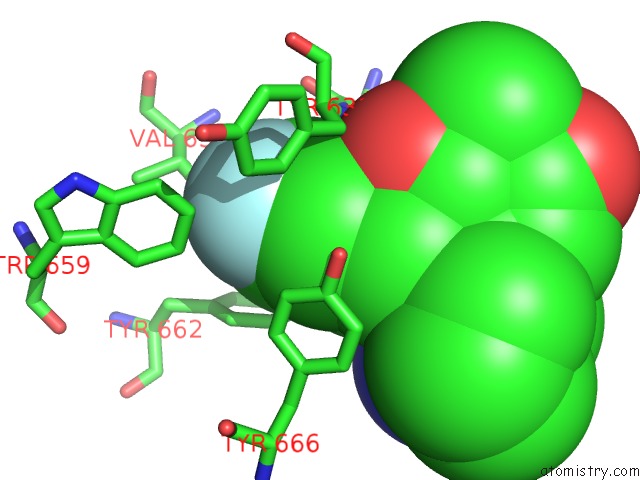
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Crystal Structure of Dipeptidyl Peptidase IV in Complex with Syr-472 within 5.0Å range:
|
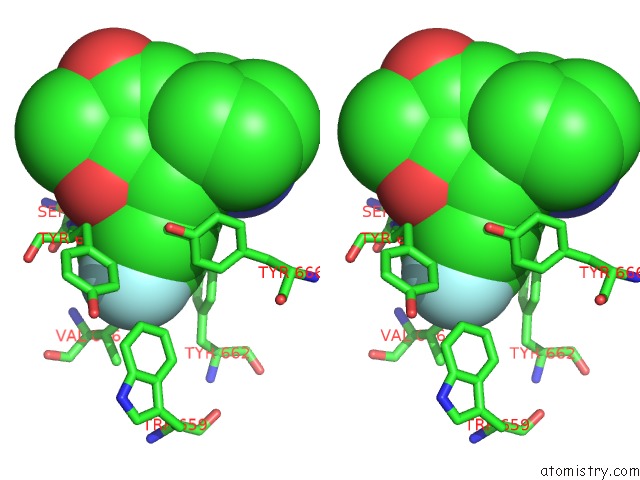
Fluorine binding site 4 out of 4 in 5kby
Go back to
Fluorine binding site 4 out
of 4 in the Crystal Structure of Dipeptidyl Peptidase IV in Complex with Syr-472
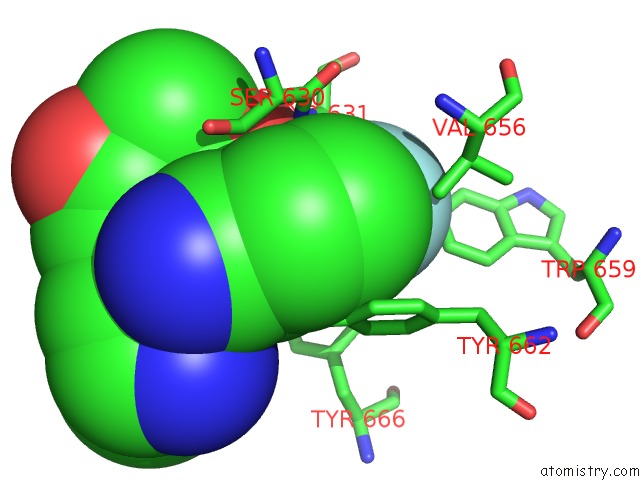
Mono view
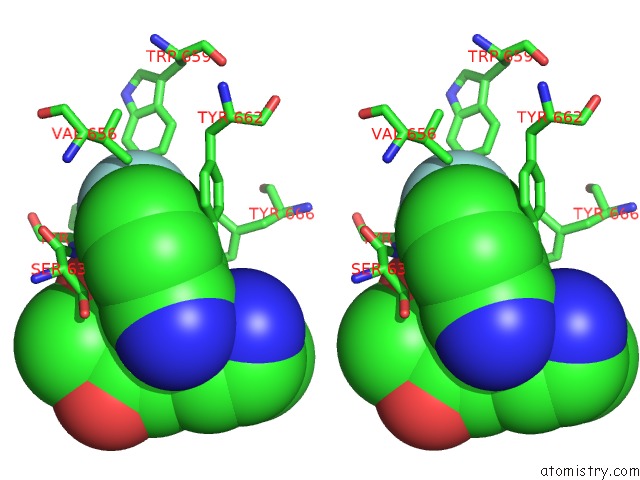
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 4 of Crystal Structure of Dipeptidyl Peptidase IV in Complex with Syr-472 within 5.0Å range:
|
Reference:
C.E.Grimshaw,
A.Jennings,
R.Kamran,
H.Ueno,
N.Nishigaki,
T.Kosaka,
A.Tani,
H.Sano,
Y.Kinugawa,
E.Koumura,
L.Shi,
K.Takeuchi.
Trelagliptin (Syr-472, Zafatek), Novel Once-Weekly Treatment For Type 2 Diabetes, Inhibits Dipeptidyl Peptidase-4 (Dpp-4) Via A Non-Covalent Mechanism. Plos One V. 11 57509 2016.
ISSN: ESSN 1932-6203
PubMed: 27328054
DOI: 10.1371/JOURNAL.PONE.0157509
Page generated: Thu Aug 1 10:57:39 2024
ISSN: ESSN 1932-6203
PubMed: 27328054
DOI: 10.1371/JOURNAL.PONE.0157509
Last articles
Ca in 5SZMCa in 5SZL
Ca in 5SY1
Ca in 5SWI
Ca in 5SVE
Ca in 5SSX
Ca in 5SV0
Ca in 5STD
Ca in 5SSZ
Ca in 5SSY