Fluorine »
PDB 5msb-5njh »
5njh »
Fluorine in PDB 5njh: Triazolopyrimidines Stabilize Microtubules By Binding to the Vinca Inhibitor Site of Tubulin
Protein crystallography data
The structure of Triazolopyrimidines Stabilize Microtubules By Binding to the Vinca Inhibitor Site of Tubulin, PDB code: 5njh
was solved by
A.Sharma,
G.S.Calvo,
A.E.Prota,
J.F.Diaz,
M.O.Steinmetz,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 48.09 / 2.39 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 103.430, 158.353, 173.458, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 21.8 / 23.7 |
Other elements in 5njh:
The structure of Triazolopyrimidines Stabilize Microtubules By Binding to the Vinca Inhibitor Site of Tubulin also contains other interesting chemical elements:
| Magnesium | (Mg) | 5 atoms |
| Chlorine | (Cl) | 1 atom |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Triazolopyrimidines Stabilize Microtubules By Binding to the Vinca Inhibitor Site of Tubulin
(pdb code 5njh). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 3 binding sites of Fluorine where determined in the Triazolopyrimidines Stabilize Microtubules By Binding to the Vinca Inhibitor Site of Tubulin, PDB code: 5njh:
Jump to Fluorine binding site number: 1; 2; 3;
In total 3 binding sites of Fluorine where determined in the Triazolopyrimidines Stabilize Microtubules By Binding to the Vinca Inhibitor Site of Tubulin, PDB code: 5njh:
Jump to Fluorine binding site number: 1; 2; 3;
Fluorine binding site 1 out of 3 in 5njh
Go back to
Fluorine binding site 1 out
of 3 in the Triazolopyrimidines Stabilize Microtubules By Binding to the Vinca Inhibitor Site of Tubulin
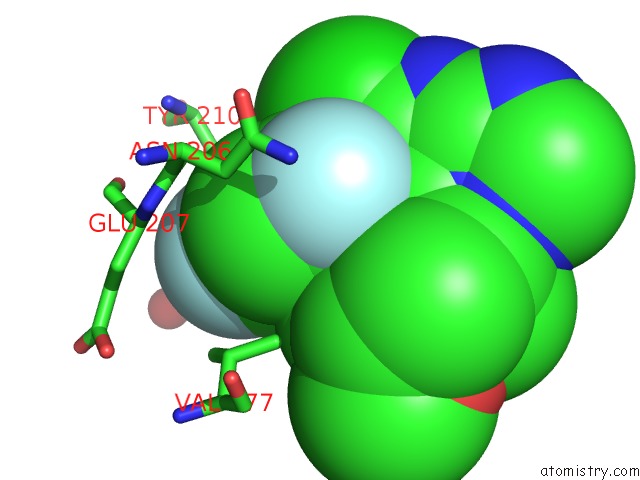
Mono view
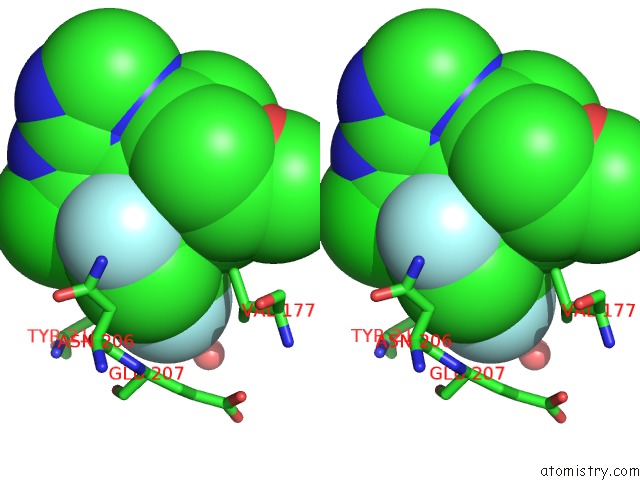
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Triazolopyrimidines Stabilize Microtubules By Binding to the Vinca Inhibitor Site of Tubulin within 5.0Å range:
|
Fluorine binding site 2 out of 3 in 5njh
Go back to
Fluorine binding site 2 out
of 3 in the Triazolopyrimidines Stabilize Microtubules By Binding to the Vinca Inhibitor Site of Tubulin
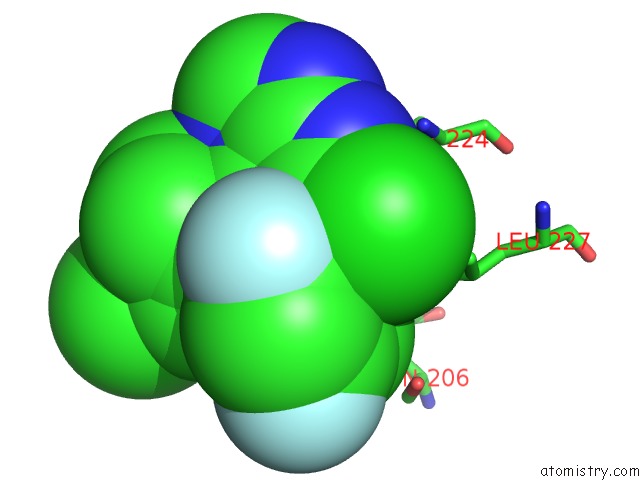
Mono view
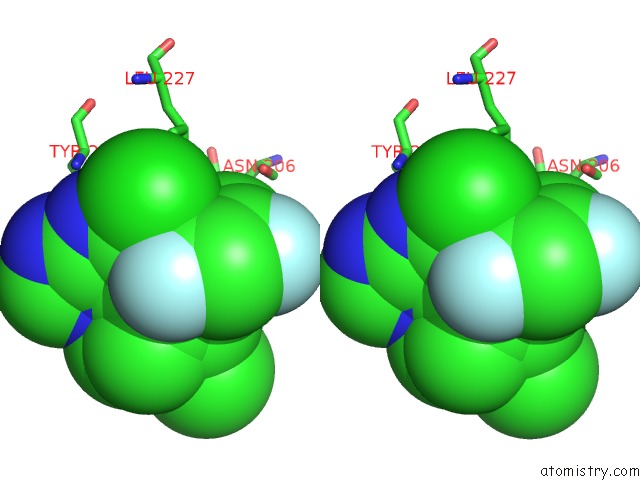
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Triazolopyrimidines Stabilize Microtubules By Binding to the Vinca Inhibitor Site of Tubulin within 5.0Å range:
|
Fluorine binding site 3 out of 3 in 5njh
Go back to
Fluorine binding site 3 out
of 3 in the Triazolopyrimidines Stabilize Microtubules By Binding to the Vinca Inhibitor Site of Tubulin
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Triazolopyrimidines Stabilize Microtubules By Binding to the Vinca Inhibitor Site of Tubulin within 5.0Å range:
|
Reference:
G.Saez-Calvo,
A.Sharma,
F.A.Balaguer,
I.Barasoain,
J.Rodriguez-Salarichs,
N.Olieric,
H.Munoz-Hernandez,
M.A.Berbis,
S.Wendeborn,
M.A.Penalva,
R.Matesanz,
A.Canales,
A.E.Prota,
J.Jimenez-Barbero,
J.M.Andreu,
C.Lamberth,
M.O.Steinmetz,
J.F.Diaz.
Triazolopyrimidines Are Microtubule-Stabilizing Agents That Bind the Vinca Inhibitor Site of Tubulin. Cell Chem Biol V. 24 737 2017.
ISSN: ESSN 2451-9456
PubMed: 28579361
DOI: 10.1016/J.CHEMBIOL.2017.05.016
Page generated: Thu Aug 1 12:00:54 2024
ISSN: ESSN 2451-9456
PubMed: 28579361
DOI: 10.1016/J.CHEMBIOL.2017.05.016
Last articles
Ca in 5ULUCa in 5UKG
Ca in 5UMH
Ca in 5UM1
Ca in 5ULV
Ca in 5ULJ
Ca in 5UK5
Ca in 5ULI
Ca in 5UKH
Ca in 5UG4