Fluorine »
PDB 5olx-5p93 »
5op4 »
Fluorine in PDB 5op4: Structure of CHK1 10-Pt. Mutant Complex with Aminopyrimidine LRRK2 Inhibitor
Enzymatic activity of Structure of CHK1 10-Pt. Mutant Complex with Aminopyrimidine LRRK2 Inhibitor
All present enzymatic activity of Structure of CHK1 10-Pt. Mutant Complex with Aminopyrimidine LRRK2 Inhibitor:
2.7.11.1;
2.7.11.1;
Protein crystallography data
The structure of Structure of CHK1 10-Pt. Mutant Complex with Aminopyrimidine LRRK2 Inhibitor, PDB code: 5op4
was solved by
P.Dokurno,
D.S.Williamson,
P.Acheson-Dossang,
I.Chen,
J.B.Murray,
T.Shaw,
A.E.Surgenor,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 20.00 / 2.00 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 45.250, 66.210, 54.610, 90.00, 102.23, 90.00 |
| R / Rfree (%) | 16.7 / 20.5 |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Structure of CHK1 10-Pt. Mutant Complex with Aminopyrimidine LRRK2 Inhibitor
(pdb code 5op4). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 4 binding sites of Fluorine where determined in the Structure of CHK1 10-Pt. Mutant Complex with Aminopyrimidine LRRK2 Inhibitor, PDB code: 5op4:
Jump to Fluorine binding site number: 1; 2; 3; 4;
In total 4 binding sites of Fluorine where determined in the Structure of CHK1 10-Pt. Mutant Complex with Aminopyrimidine LRRK2 Inhibitor, PDB code: 5op4:
Jump to Fluorine binding site number: 1; 2; 3; 4;
Fluorine binding site 1 out of 4 in 5op4
Go back to
Fluorine binding site 1 out
of 4 in the Structure of CHK1 10-Pt. Mutant Complex with Aminopyrimidine LRRK2 Inhibitor
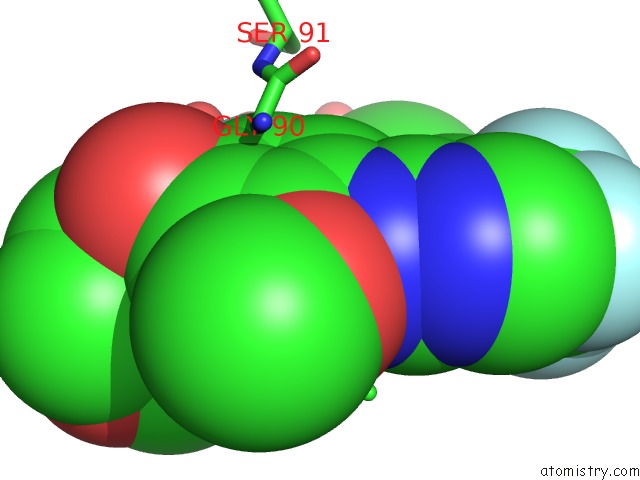
Mono view
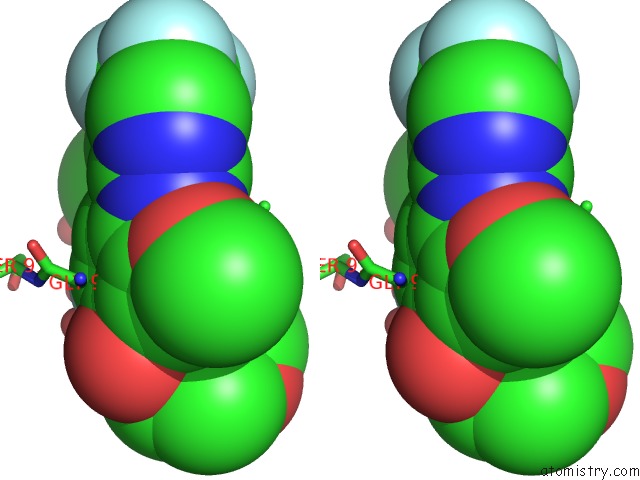
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Structure of CHK1 10-Pt. Mutant Complex with Aminopyrimidine LRRK2 Inhibitor within 5.0Å range:
|
Fluorine binding site 2 out of 4 in 5op4
Go back to
Fluorine binding site 2 out
of 4 in the Structure of CHK1 10-Pt. Mutant Complex with Aminopyrimidine LRRK2 Inhibitor
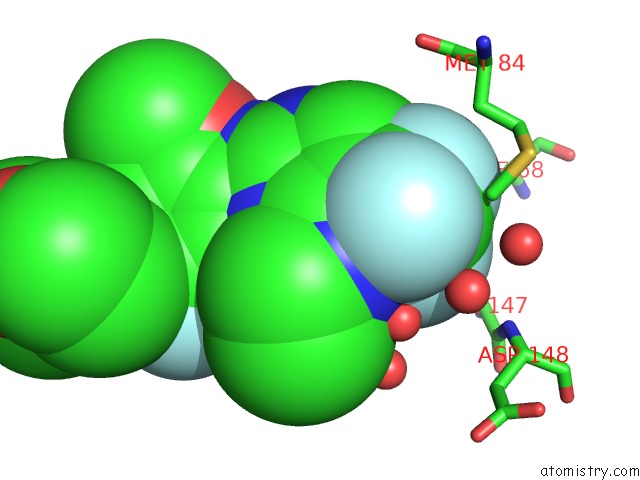
Mono view
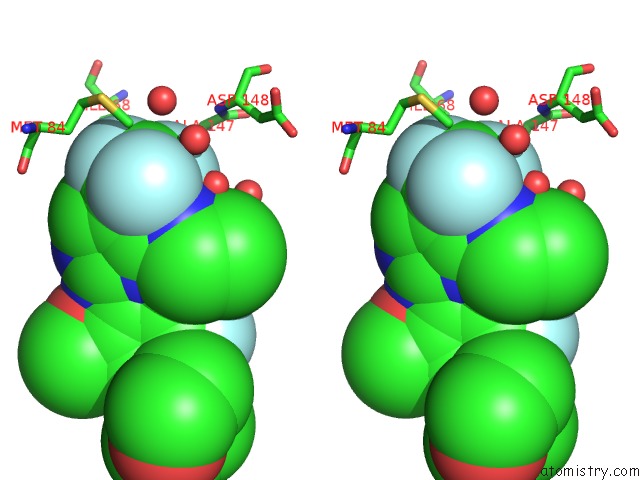
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Structure of CHK1 10-Pt. Mutant Complex with Aminopyrimidine LRRK2 Inhibitor within 5.0Å range:
|
Fluorine binding site 3 out of 4 in 5op4
Go back to
Fluorine binding site 3 out
of 4 in the Structure of CHK1 10-Pt. Mutant Complex with Aminopyrimidine LRRK2 Inhibitor
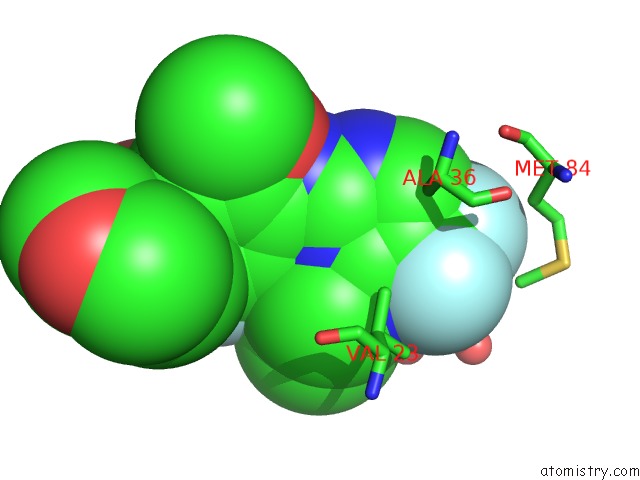
Mono view
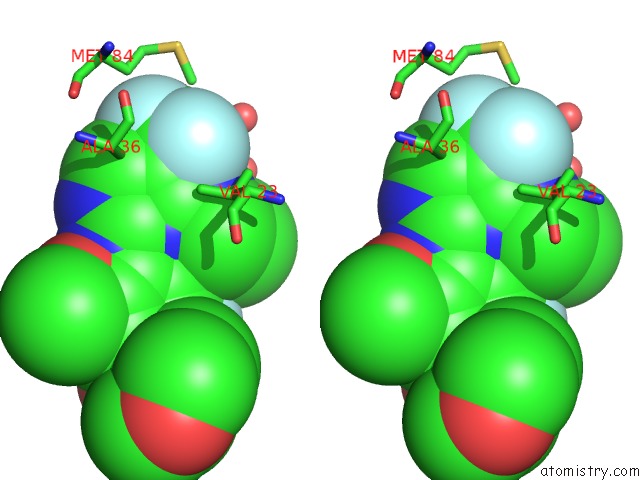
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Structure of CHK1 10-Pt. Mutant Complex with Aminopyrimidine LRRK2 Inhibitor within 5.0Å range:
|
Fluorine binding site 4 out of 4 in 5op4
Go back to
Fluorine binding site 4 out
of 4 in the Structure of CHK1 10-Pt. Mutant Complex with Aminopyrimidine LRRK2 Inhibitor
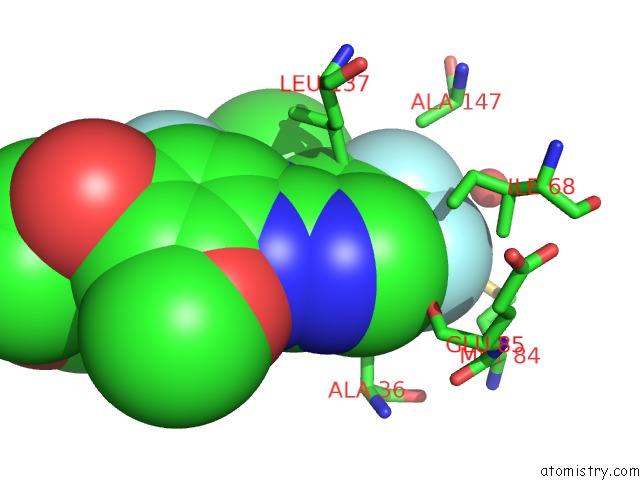
Mono view
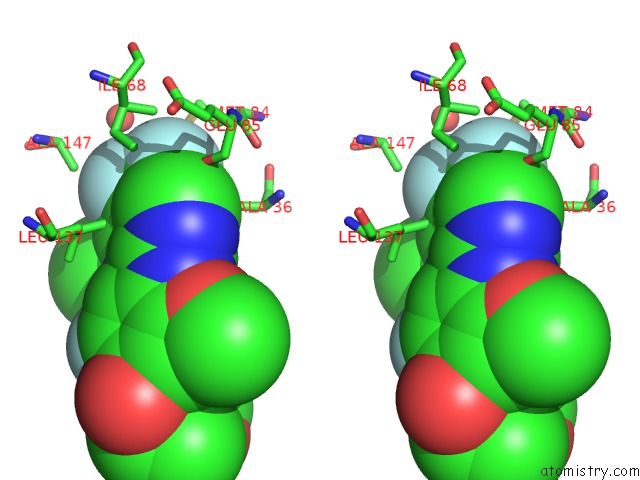
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 4 of Structure of CHK1 10-Pt. Mutant Complex with Aminopyrimidine LRRK2 Inhibitor within 5.0Å range:
|
Reference:
D.S.Williamson,
G.P.Smith,
P.Acheson-Dossang,
S.T.Bedford,
V.Chell,
I.J.Chen,
J.C.A.Daechsel,
Z.Daniels,
L.David,
P.Dokurno,
M.Hentzer,
M.C.Herzig,
R.E.Hubbard,
J.D.Moore,
J.B.Murray,
S.Newland,
S.C.Ray,
T.Shaw,
A.E.Surgenor,
L.Terry,
K.Thirstrup,
Y.Wang,
K.V.Christensen.
Design of Leucine-Rich Repeat Kinase 2 (LRRK2) Inhibitors Using A Crystallographic Surrogate Derived From Checkpoint Kinase 1 (CHK1). J. Med. Chem. V. 60 8945 2017.
ISSN: ISSN 1520-4804
PubMed: 29023112
DOI: 10.1021/ACS.JMEDCHEM.7B01186
Page generated: Tue Jul 15 05:52:00 2025
ISSN: ISSN 1520-4804
PubMed: 29023112
DOI: 10.1021/ACS.JMEDCHEM.7B01186
Last articles
F in 6EL6F in 6EL5
F in 6EHU
F in 6EKH
F in 6EE2
F in 6EIM
F in 6EI4
F in 6EEH
F in 6EGA
F in 6EG9