Fluorine »
PDB 5xd5-5y5r »
5xl0 »
Fluorine in PDB 5xl0: Met-Aquo Form of Sperm Whale Myoglobin Reconstituted with 7-Pf, A Heme Possesseing CF3 Group As Side Chain
Protein crystallography data
The structure of Met-Aquo Form of Sperm Whale Myoglobin Reconstituted with 7-Pf, A Heme Possesseing CF3 Group As Side Chain, PDB code: 5xl0
was solved by
Y.Kanai,
A.Harada,
T.Shibata,
R.Nishimura,
K.Namiki,
M.Watanabe,
S.Nakamura,
F.Yumoto,
T.Senda,
A.Suzuki,
S.Neya,
Y.Yamamoto,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 30.92 / 1.25 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 34.261, 30.854, 64.130, 90.00, 105.36, 90.00 |
| R / Rfree (%) | 16.3 / 17.3 |
Other elements in 5xl0:
The structure of Met-Aquo Form of Sperm Whale Myoglobin Reconstituted with 7-Pf, A Heme Possesseing CF3 Group As Side Chain also contains other interesting chemical elements:
| Iron | (Fe) | 2 atoms |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Met-Aquo Form of Sperm Whale Myoglobin Reconstituted with 7-Pf, A Heme Possesseing CF3 Group As Side Chain
(pdb code 5xl0). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 6 binding sites of Fluorine where determined in the Met-Aquo Form of Sperm Whale Myoglobin Reconstituted with 7-Pf, A Heme Possesseing CF3 Group As Side Chain, PDB code: 5xl0:
Jump to Fluorine binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Fluorine where determined in the Met-Aquo Form of Sperm Whale Myoglobin Reconstituted with 7-Pf, A Heme Possesseing CF3 Group As Side Chain, PDB code: 5xl0:
Jump to Fluorine binding site number: 1; 2; 3; 4; 5; 6;
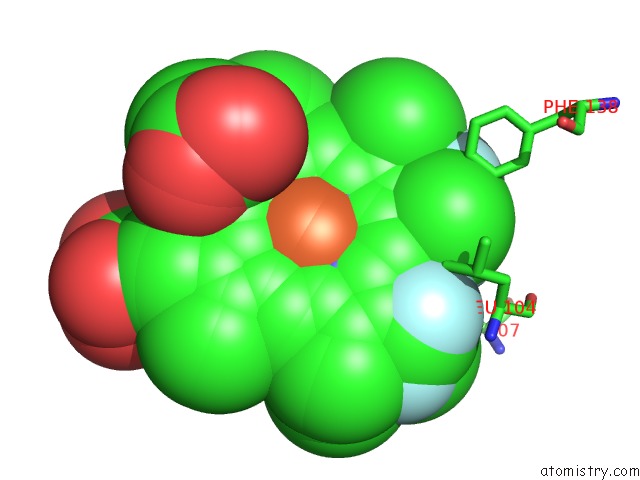
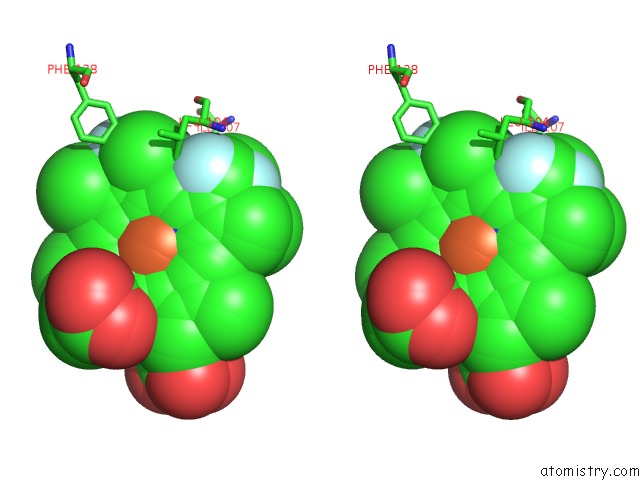
Fluorine binding site 1 out of 6 in 5xl0
Go back to
Fluorine binding site 1 out
of 6 in the Met-Aquo Form of Sperm Whale Myoglobin Reconstituted with 7-Pf, A Heme Possesseing CF3 Group As Side Chain
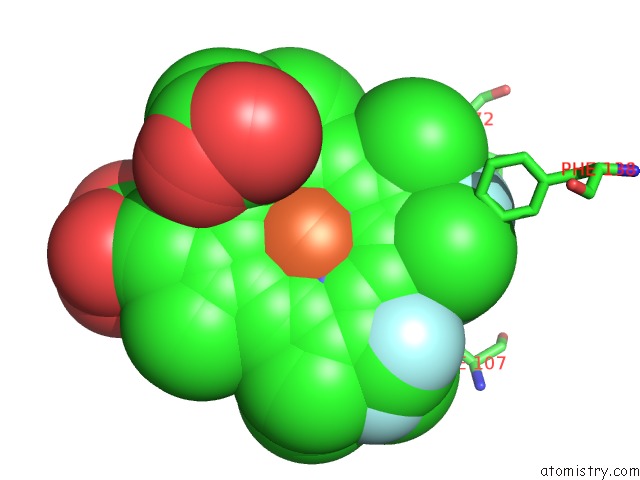
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Met-Aquo Form of Sperm Whale Myoglobin Reconstituted with 7-Pf, A Heme Possesseing CF3 Group As Side Chain within 5.0Å range:
|
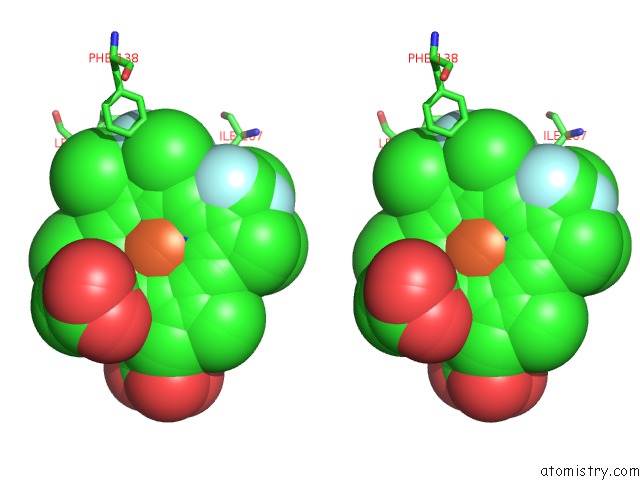
Fluorine binding site 2 out of 6 in 5xl0
Go back to
Fluorine binding site 2 out
of 6 in the Met-Aquo Form of Sperm Whale Myoglobin Reconstituted with 7-Pf, A Heme Possesseing CF3 Group As Side Chain
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Met-Aquo Form of Sperm Whale Myoglobin Reconstituted with 7-Pf, A Heme Possesseing CF3 Group As Side Chain within 5.0Å range:
|
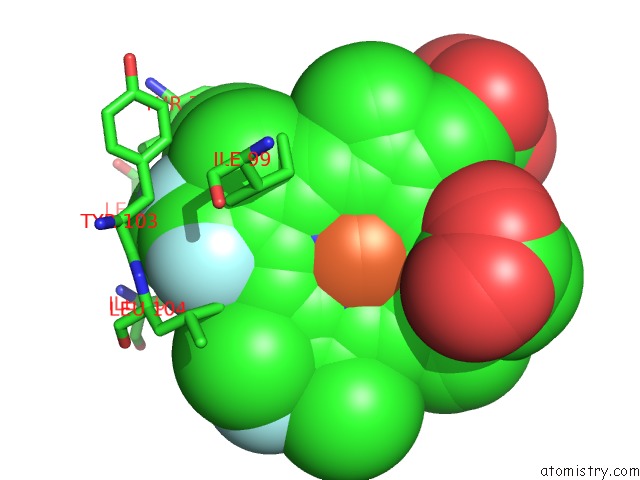
Fluorine binding site 3 out of 6 in 5xl0
Go back to
Fluorine binding site 3 out
of 6 in the Met-Aquo Form of Sperm Whale Myoglobin Reconstituted with 7-Pf, A Heme Possesseing CF3 Group As Side Chain
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Met-Aquo Form of Sperm Whale Myoglobin Reconstituted with 7-Pf, A Heme Possesseing CF3 Group As Side Chain within 5.0Å range:
|
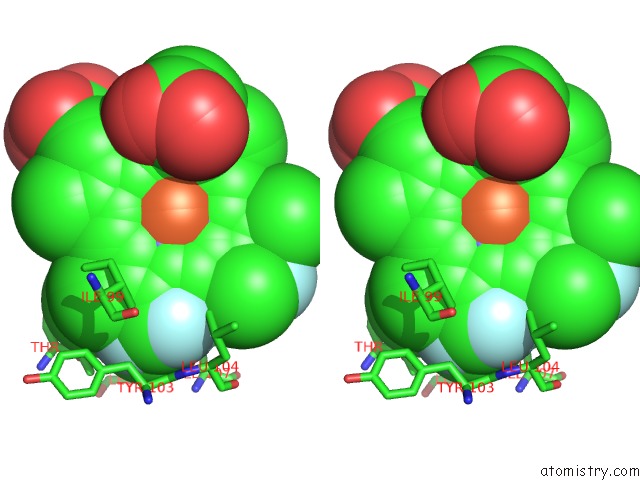
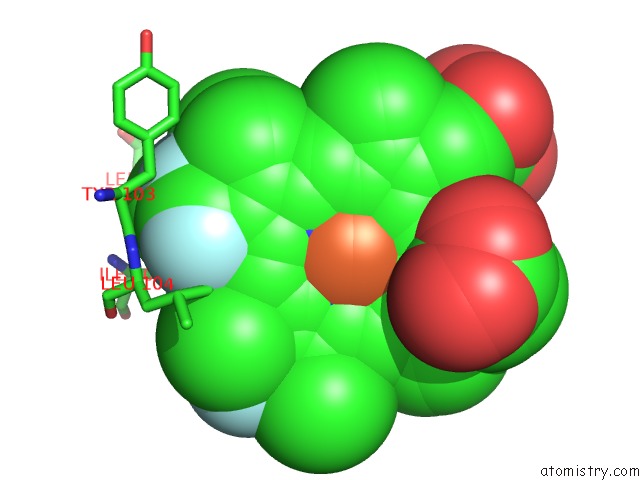
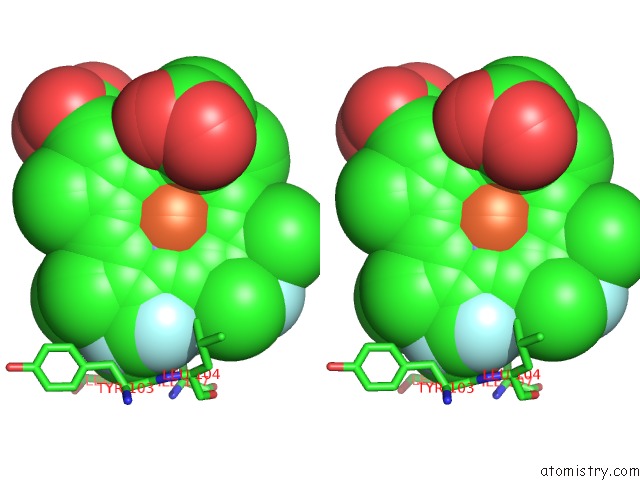
Fluorine binding site 4 out of 6 in 5xl0
Go back to
Fluorine binding site 4 out
of 6 in the Met-Aquo Form of Sperm Whale Myoglobin Reconstituted with 7-Pf, A Heme Possesseing CF3 Group As Side Chain
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 4 of Met-Aquo Form of Sperm Whale Myoglobin Reconstituted with 7-Pf, A Heme Possesseing CF3 Group As Side Chain within 5.0Å range:
|
Fluorine binding site 5 out of 6 in 5xl0
Go back to
Fluorine binding site 5 out
of 6 in the Met-Aquo Form of Sperm Whale Myoglobin Reconstituted with 7-Pf, A Heme Possesseing CF3 Group As Side Chain
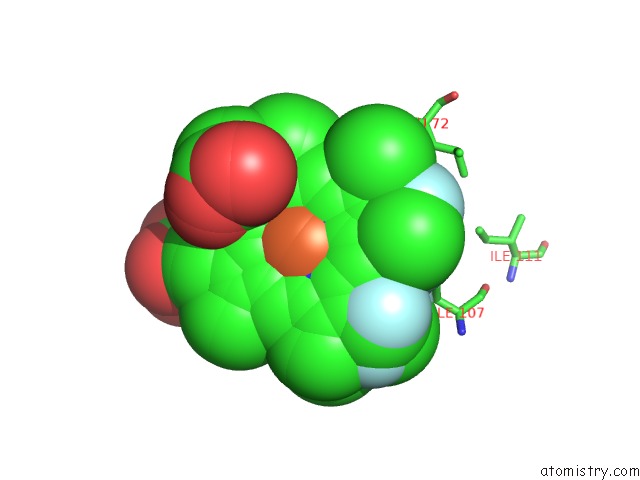
Mono view
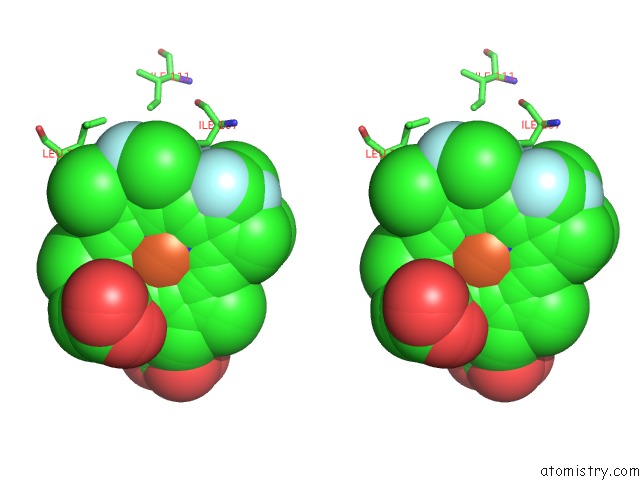
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 5 of Met-Aquo Form of Sperm Whale Myoglobin Reconstituted with 7-Pf, A Heme Possesseing CF3 Group As Side Chain within 5.0Å range:
|
Fluorine binding site 6 out of 6 in 5xl0
Go back to
Fluorine binding site 6 out
of 6 in the Met-Aquo Form of Sperm Whale Myoglobin Reconstituted with 7-Pf, A Heme Possesseing CF3 Group As Side Chain
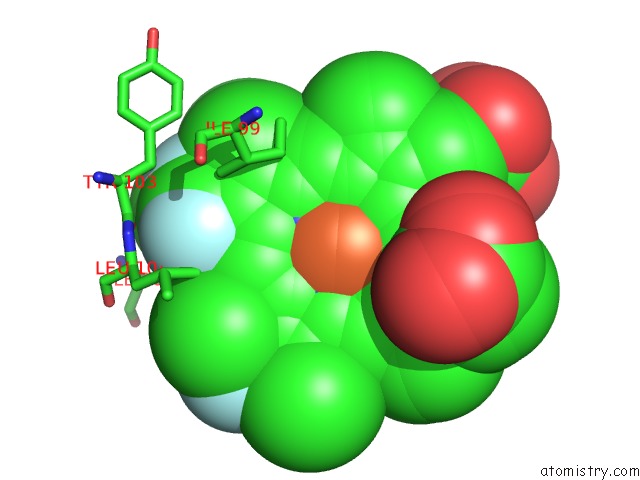
Mono view
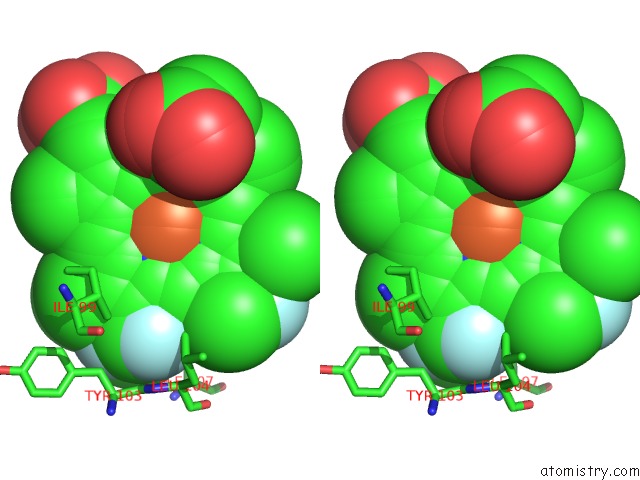
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 6 of Met-Aquo Form of Sperm Whale Myoglobin Reconstituted with 7-Pf, A Heme Possesseing CF3 Group As Side Chain within 5.0Å range:
|
Reference:
Y.Kanai,
A.Harada,
T.Shibata,
R.Nishimura,
K.Namiki,
M.Watanabe,
S.Nakamura,
F.Yumoto,
T.Senda,
A.Suzuki,
S.Neya,
Y.Yamamoto.
Characterization of Heme Orientational Disorder in A Myoglobin Reconstituted with A Trifluoromethyl-Group-Substituted Heme Cofactor Biochemistry V. 56 4500 2017.
ISSN: ISSN 1520-4995
PubMed: 28758387
DOI: 10.1021/ACS.BIOCHEM.7B00457
Page generated: Thu Aug 1 16:55:35 2024
ISSN: ISSN 1520-4995
PubMed: 28758387
DOI: 10.1021/ACS.BIOCHEM.7B00457
Last articles
Zn in 9MJ5Zn in 9HNW
Zn in 9G0L
Zn in 9FNE
Zn in 9DZN
Zn in 9E0I
Zn in 9D32
Zn in 9DAK
Zn in 8ZXC
Zn in 8ZUF