Fluorine »
PDB 6dmg-6e69 »
6e5g »
Fluorine in PDB 6e5g: Crystal Structure of Human TEAD2-Yap Binding Domain Covalently Bound to An Allosteric Regulator
Protein crystallography data
The structure of Crystal Structure of Human TEAD2-Yap Binding Domain Covalently Bound to An Allosteric Regulator, PDB code: 6e5g
was solved by
K.Bum-Erdene,
G.Gonzalez-Gutierrez,
S.O.Meroueh,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 38.89 / 2.43 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 123.757, 61.226, 79.839, 90.00, 116.89, 90.00 |
| R / Rfree (%) | 22.1 / 26.8 |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Crystal Structure of Human TEAD2-Yap Binding Domain Covalently Bound to An Allosteric Regulator
(pdb code 6e5g). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 6 binding sites of Fluorine where determined in the Crystal Structure of Human TEAD2-Yap Binding Domain Covalently Bound to An Allosteric Regulator, PDB code: 6e5g:
Jump to Fluorine binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Fluorine where determined in the Crystal Structure of Human TEAD2-Yap Binding Domain Covalently Bound to An Allosteric Regulator, PDB code: 6e5g:
Jump to Fluorine binding site number: 1; 2; 3; 4; 5; 6;
Fluorine binding site 1 out of 6 in 6e5g
Go back to
Fluorine binding site 1 out
of 6 in the Crystal Structure of Human TEAD2-Yap Binding Domain Covalently Bound to An Allosteric Regulator
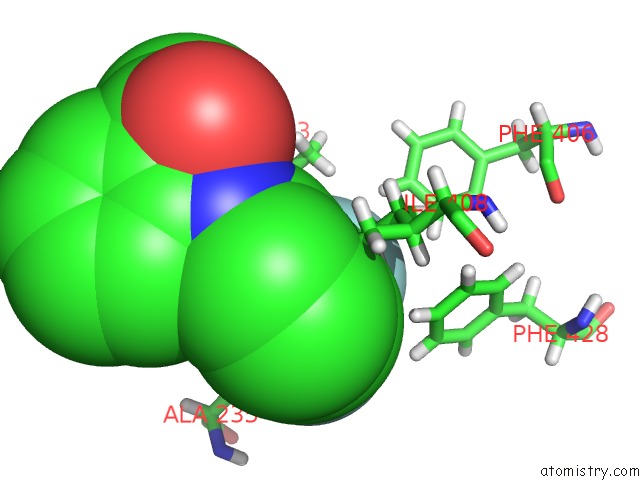
Mono view
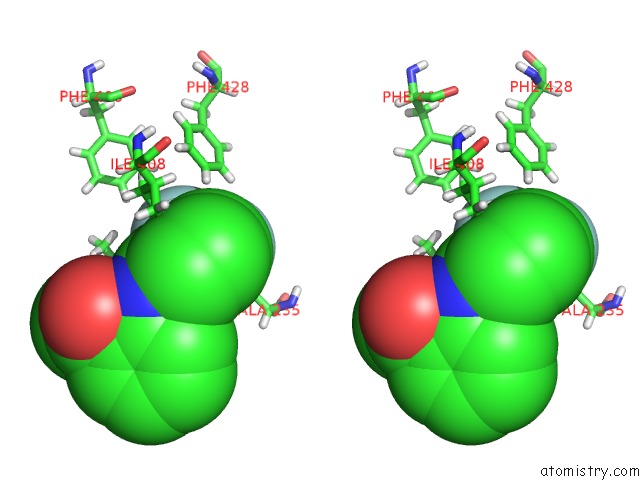
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Crystal Structure of Human TEAD2-Yap Binding Domain Covalently Bound to An Allosteric Regulator within 5.0Å range:
|
Fluorine binding site 2 out of 6 in 6e5g
Go back to
Fluorine binding site 2 out
of 6 in the Crystal Structure of Human TEAD2-Yap Binding Domain Covalently Bound to An Allosteric Regulator
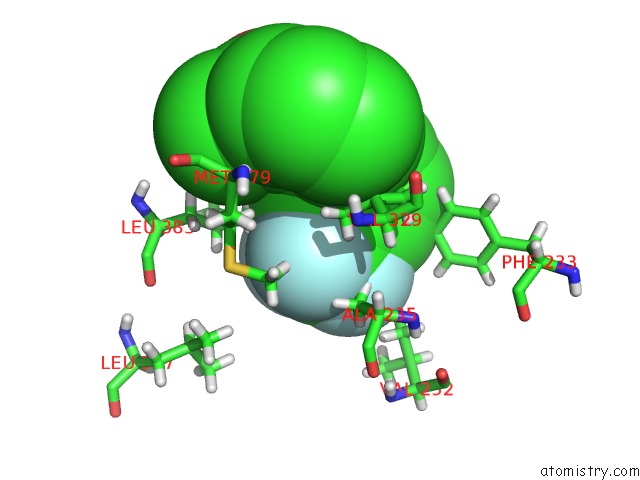
Mono view
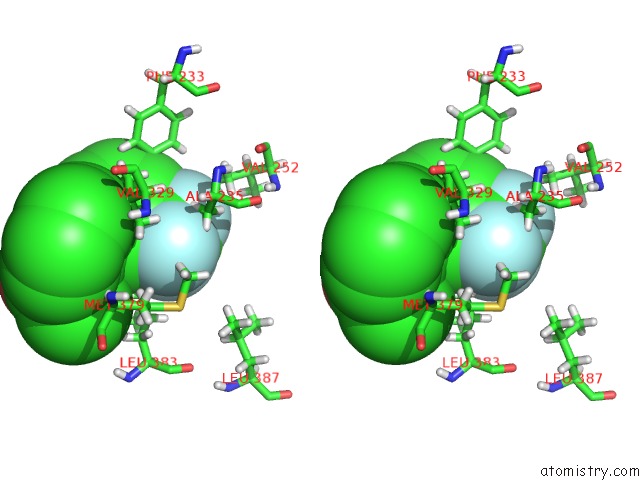
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Crystal Structure of Human TEAD2-Yap Binding Domain Covalently Bound to An Allosteric Regulator within 5.0Å range:
|
Fluorine binding site 3 out of 6 in 6e5g
Go back to
Fluorine binding site 3 out
of 6 in the Crystal Structure of Human TEAD2-Yap Binding Domain Covalently Bound to An Allosteric Regulator
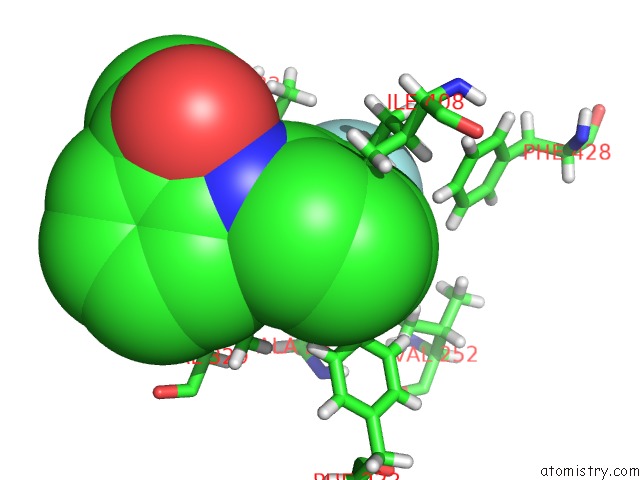
Mono view
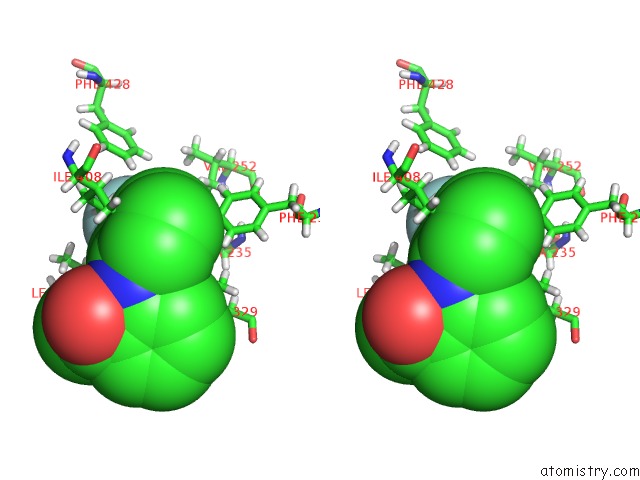
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Crystal Structure of Human TEAD2-Yap Binding Domain Covalently Bound to An Allosteric Regulator within 5.0Å range:
|
Fluorine binding site 4 out of 6 in 6e5g
Go back to
Fluorine binding site 4 out
of 6 in the Crystal Structure of Human TEAD2-Yap Binding Domain Covalently Bound to An Allosteric Regulator
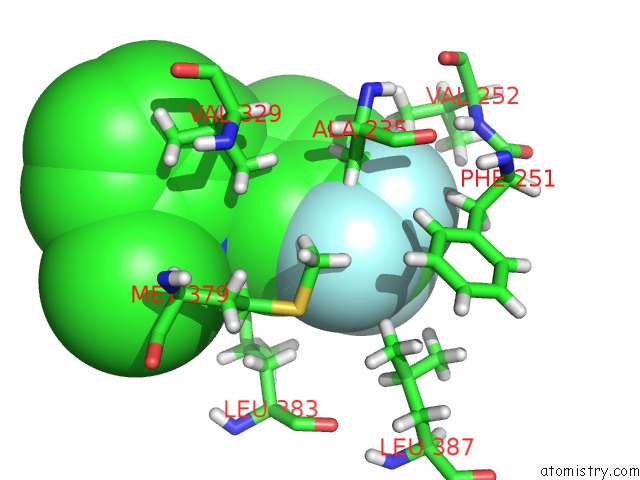
Mono view
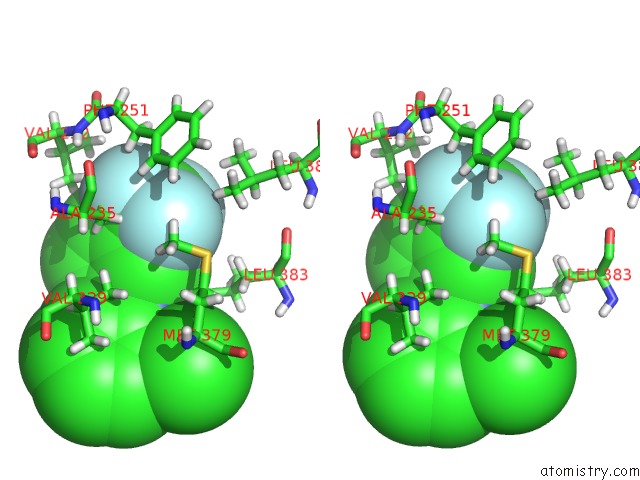
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 4 of Crystal Structure of Human TEAD2-Yap Binding Domain Covalently Bound to An Allosteric Regulator within 5.0Å range:
|
Fluorine binding site 5 out of 6 in 6e5g
Go back to
Fluorine binding site 5 out
of 6 in the Crystal Structure of Human TEAD2-Yap Binding Domain Covalently Bound to An Allosteric Regulator
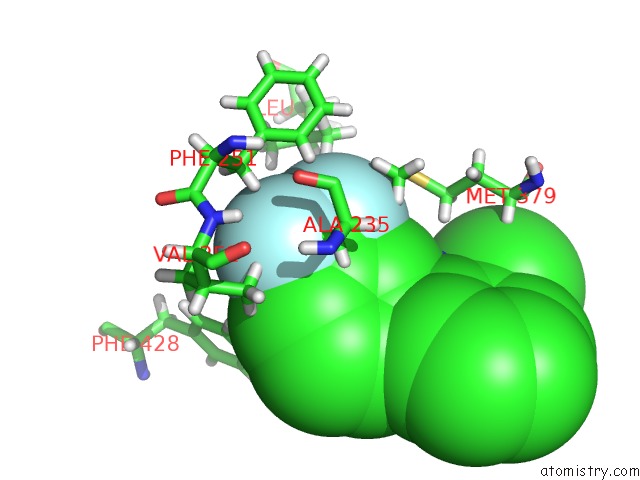
Mono view
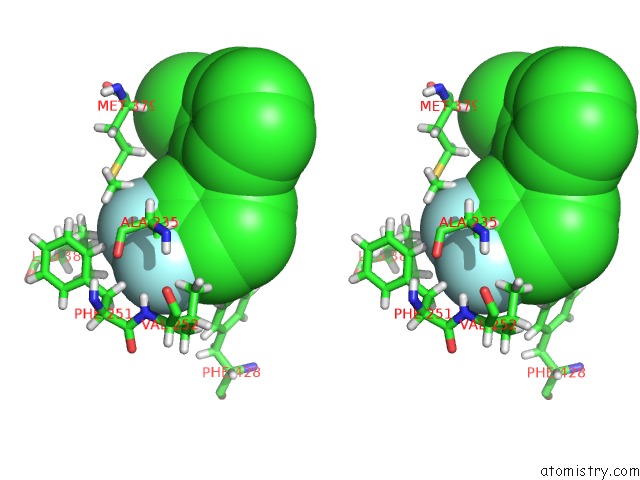
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 5 of Crystal Structure of Human TEAD2-Yap Binding Domain Covalently Bound to An Allosteric Regulator within 5.0Å range:
|
Fluorine binding site 6 out of 6 in 6e5g
Go back to
Fluorine binding site 6 out
of 6 in the Crystal Structure of Human TEAD2-Yap Binding Domain Covalently Bound to An Allosteric Regulator
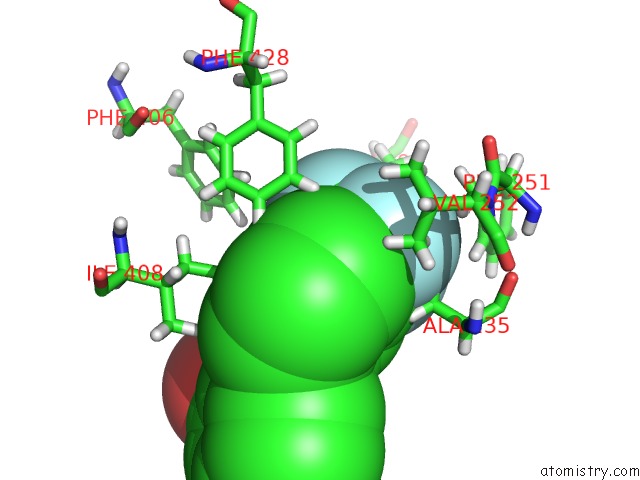
Mono view
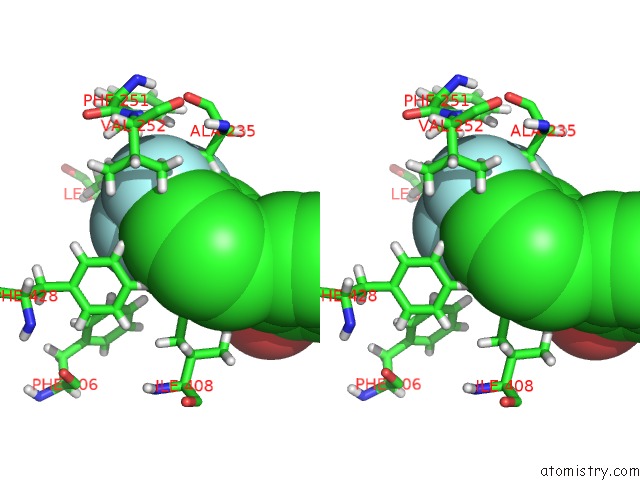
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 6 of Crystal Structure of Human TEAD2-Yap Binding Domain Covalently Bound to An Allosteric Regulator within 5.0Å range:
|
Reference:
K.Bum-Erdene,
D.Zhou,
G.Gonzalez-Gutierrez,
M.K.Ghozayel,
Y.Si,
D.Xu,
H.E.Shannon,
B.J.Bailey,
T.W.Corson,
K.E.Pollok,
C.D.Wells,
S.O.Meroueh.
Small-Molecule Covalent Modification of Conserved Cysteine Leads to Allosteric Inhibition of the Tead⋅Yap Protein-Protein Interaction. Cell Chem Biol V. 26 378 2019.
ISSN: ESSN 2451-9448
PubMed: 30581134
DOI: 10.1016/J.CHEMBIOL.2018.11.010
Page generated: Tue Jul 15 11:00:27 2025
ISSN: ESSN 2451-9448
PubMed: 30581134
DOI: 10.1016/J.CHEMBIOL.2018.11.010
Last articles
F in 6YM4F in 6YL4
F in 6YIZ
F in 6YKU
F in 6YJM
F in 6YKE
F in 6YHD
F in 6YHE
F in 6YIL
F in 6YHC