Fluorine »
PDB 6e6j-6eog »
6ea1 »
Fluorine in PDB 6ea1: X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6DA) and Catalytic Zinc Ion
Protein crystallography data
The structure of X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6DA) and Catalytic Zinc Ion, PDB code: 6ea1
was solved by
N.Drinkwater,
S.Mcgowan,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 54.46 / 1.82 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 75.491, 108.920, 118.150, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 15.7 / 20.1 |
Other elements in 6ea1:
The structure of X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6DA) and Catalytic Zinc Ion also contains other interesting chemical elements:
| Magnesium | (Mg) | 4 atoms |
| Zinc | (Zn) | 1 atom |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6DA) and Catalytic Zinc Ion
(pdb code 6ea1). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 7 binding sites of Fluorine where determined in the X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6DA) and Catalytic Zinc Ion, PDB code: 6ea1:
Jump to Fluorine binding site number: 1; 2; 3; 4; 5; 6; 7;
In total 7 binding sites of Fluorine where determined in the X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6DA) and Catalytic Zinc Ion, PDB code: 6ea1:
Jump to Fluorine binding site number: 1; 2; 3; 4; 5; 6; 7;
Fluorine binding site 1 out of 7 in 6ea1
Go back to
Fluorine binding site 1 out
of 7 in the X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6DA) and Catalytic Zinc Ion
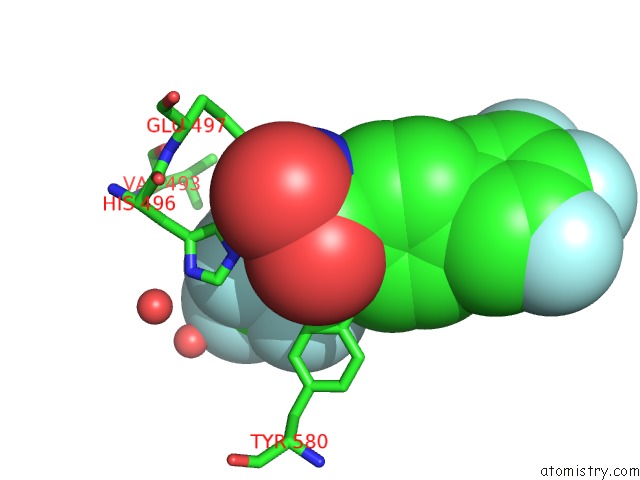
Mono view
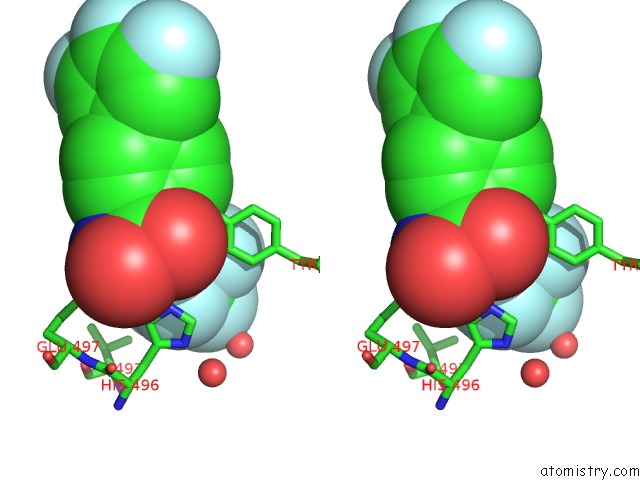
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6DA) and Catalytic Zinc Ion within 5.0Å range:
|
Fluorine binding site 2 out of 7 in 6ea1
Go back to
Fluorine binding site 2 out
of 7 in the X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6DA) and Catalytic Zinc Ion
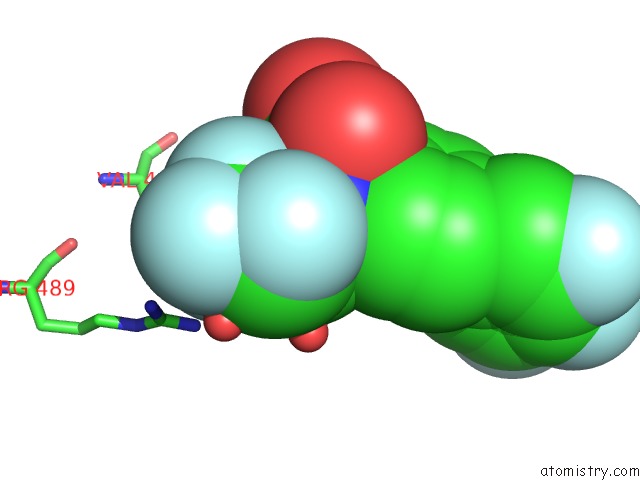
Mono view
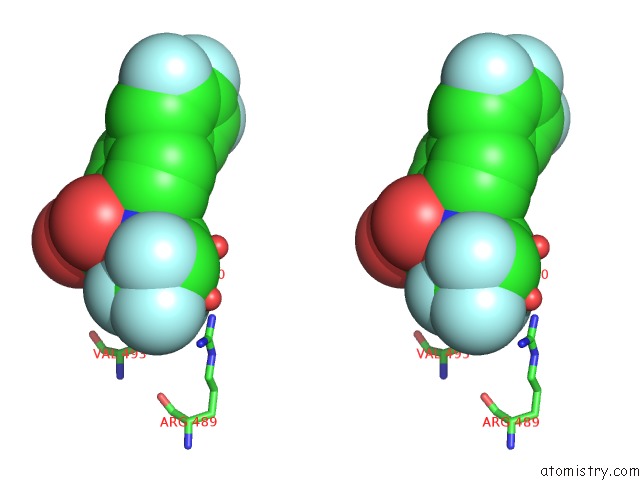
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6DA) and Catalytic Zinc Ion within 5.0Å range:
|
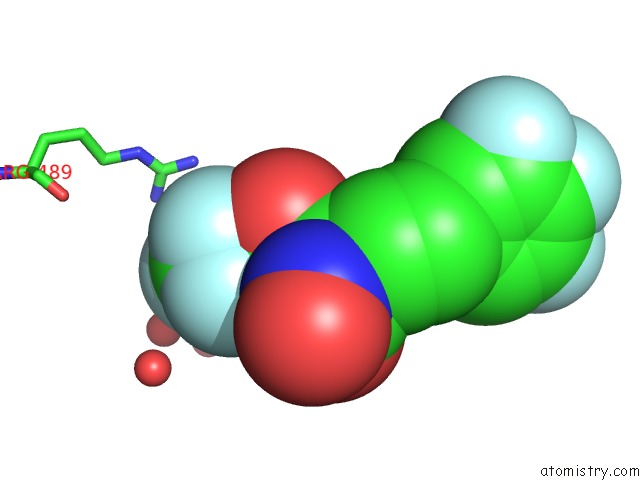
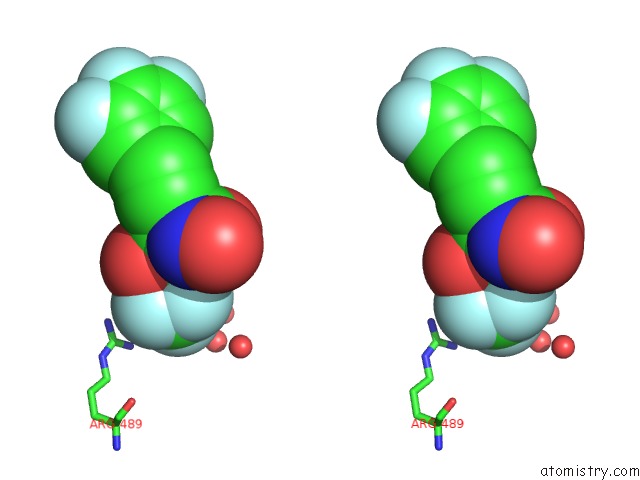
Fluorine binding site 3 out of 7 in 6ea1
Go back to
Fluorine binding site 3 out
of 7 in the X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6DA) and Catalytic Zinc Ion
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6DA) and Catalytic Zinc Ion within 5.0Å range:
|
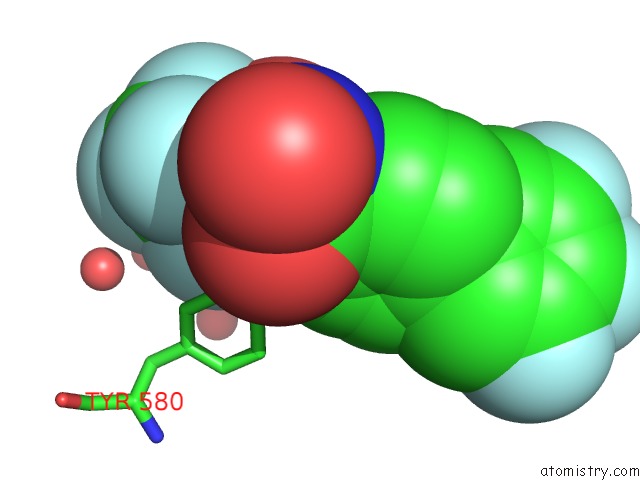
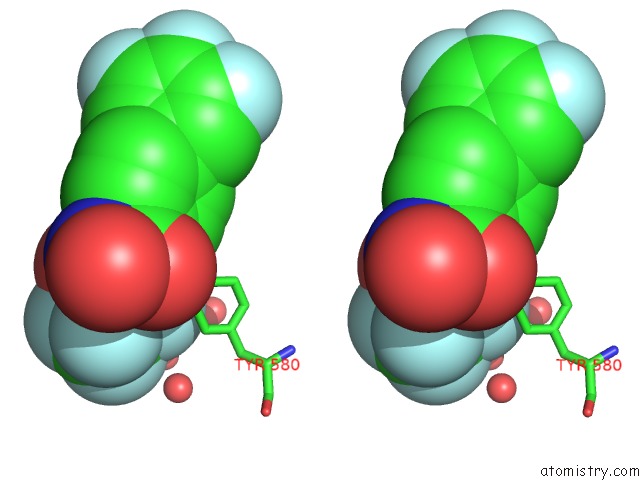
Fluorine binding site 4 out of 7 in 6ea1
Go back to
Fluorine binding site 4 out
of 7 in the X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6DA) and Catalytic Zinc Ion
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 4 of X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6DA) and Catalytic Zinc Ion within 5.0Å range:
|
Fluorine binding site 5 out of 7 in 6ea1
Go back to
Fluorine binding site 5 out
of 7 in the X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6DA) and Catalytic Zinc Ion
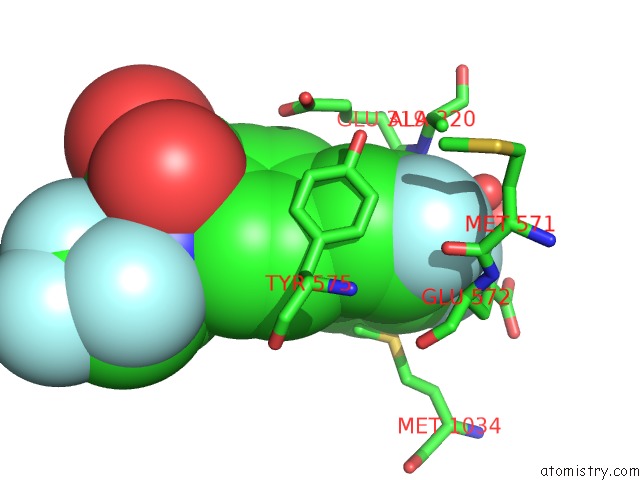
Mono view
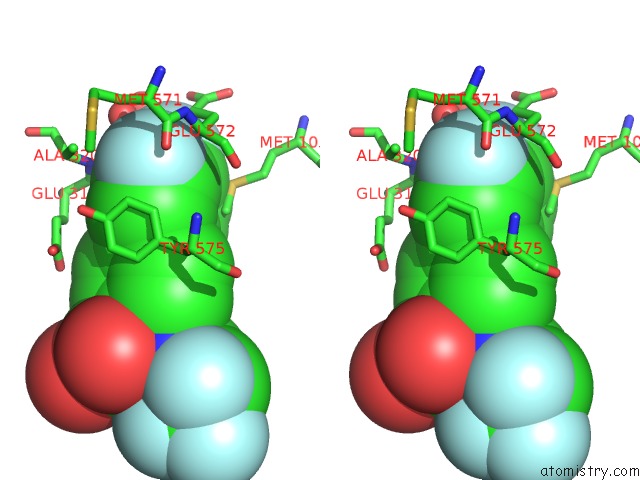
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 5 of X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6DA) and Catalytic Zinc Ion within 5.0Å range:
|
Fluorine binding site 6 out of 7 in 6ea1
Go back to
Fluorine binding site 6 out
of 7 in the X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6DA) and Catalytic Zinc Ion
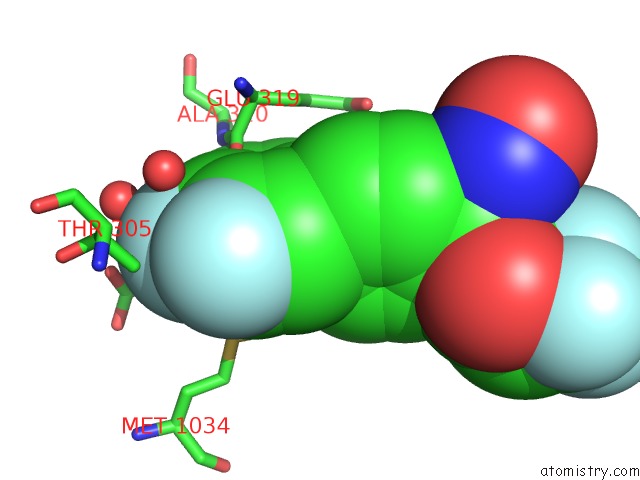
Mono view
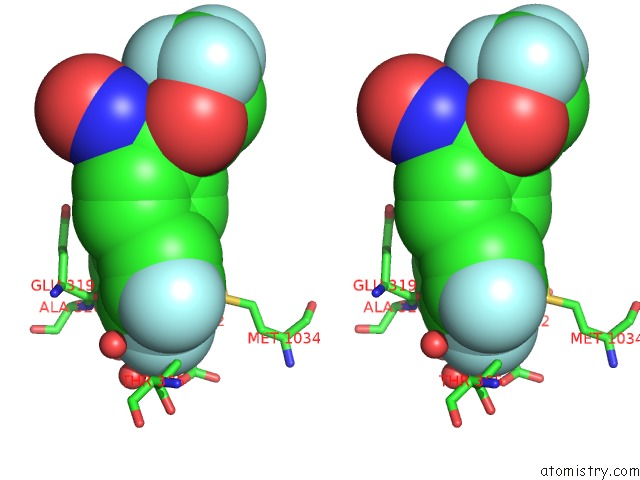
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 6 of X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6DA) and Catalytic Zinc Ion within 5.0Å range:
|
Fluorine binding site 7 out of 7 in 6ea1
Go back to
Fluorine binding site 7 out
of 7 in the X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6DA) and Catalytic Zinc Ion
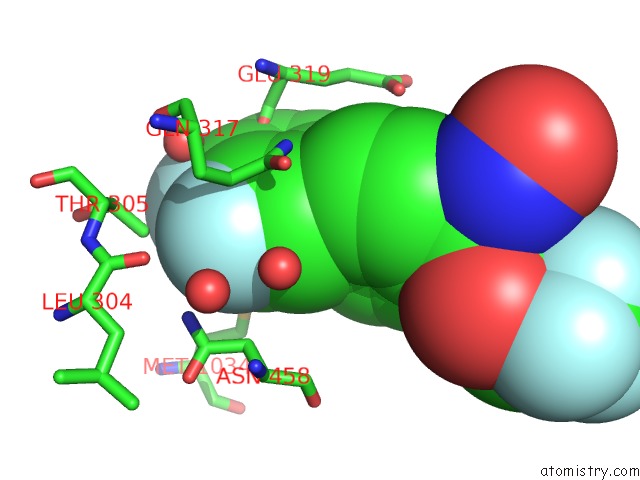
Mono view
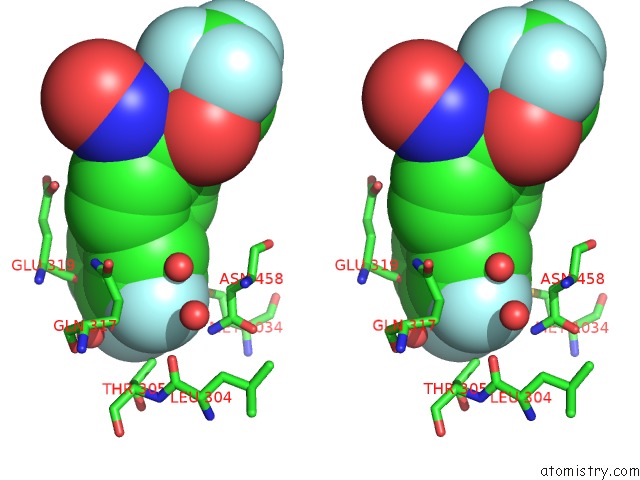
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 7 of X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6DA) and Catalytic Zinc Ion within 5.0Å range:
|
Reference:
N.B.Vinh,
N.Drinkwater,
T.R.Malcolm,
M.Kassiou,
L.Lucantoni,
P.M.Grin,
G.S.Butler,
S.Duffy,
C.M.Overall,
V.M.Avery,
P.J.Scammells,
S.Mcgowan.
Hydroxamic Acid Inhibitors Provide Cross-Species Inhibition of Plasmodium M1 and M17 Aminopeptidases. J. Med. Chem. V. 62 622 2019.
ISSN: ISSN 1520-4804
PubMed: 30537832
DOI: 10.1021/ACS.JMEDCHEM.8B01310
Page generated: Tue Jul 15 11:03:04 2025
ISSN: ISSN 1520-4804
PubMed: 30537832
DOI: 10.1021/ACS.JMEDCHEM.8B01310
Last articles
Fe in 2B11Fe in 2B12
Fe in 2B10
Fe in 2AYS
Fe in 2B0Z
Fe in 2AZQ
Fe in 2AXT
Fe in 2AV8
Fe in 2AWY
Fe in 2AXX