Fluorine »
PDB 6e69-6elo »
6eaa »
Fluorine in PDB 6eaa: X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6I) and Catalytic Zinc Ion
Protein crystallography data
The structure of X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6I) and Catalytic Zinc Ion, PDB code: 6eaa
was solved by
N.Drinkwater,
S.Mcgowan,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 46.40 / 1.65 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 75.399, 108.850, 117.720, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 15.1 / 18.3 |
Other elements in 6eaa:
The structure of X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6I) and Catalytic Zinc Ion also contains other interesting chemical elements:
| Magnesium | (Mg) | 3 atoms |
| Zinc | (Zn) | 1 atom |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6I) and Catalytic Zinc Ion
(pdb code 6eaa). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 3 binding sites of Fluorine where determined in the X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6I) and Catalytic Zinc Ion, PDB code: 6eaa:
Jump to Fluorine binding site number: 1; 2; 3;
In total 3 binding sites of Fluorine where determined in the X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6I) and Catalytic Zinc Ion, PDB code: 6eaa:
Jump to Fluorine binding site number: 1; 2; 3;
Fluorine binding site 1 out of 3 in 6eaa
Go back to
Fluorine binding site 1 out
of 3 in the X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6I) and Catalytic Zinc Ion
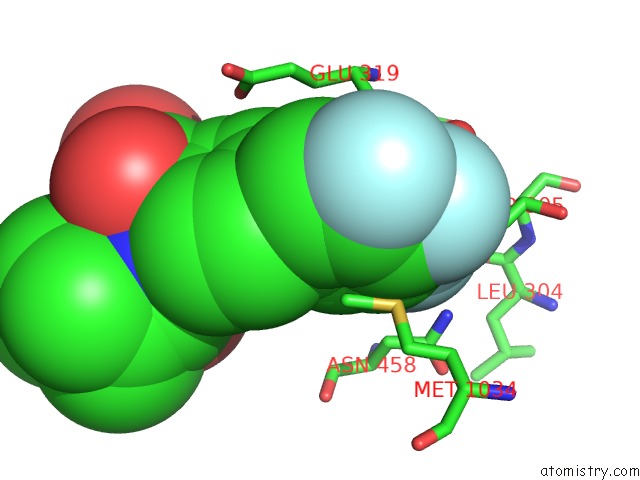
Mono view
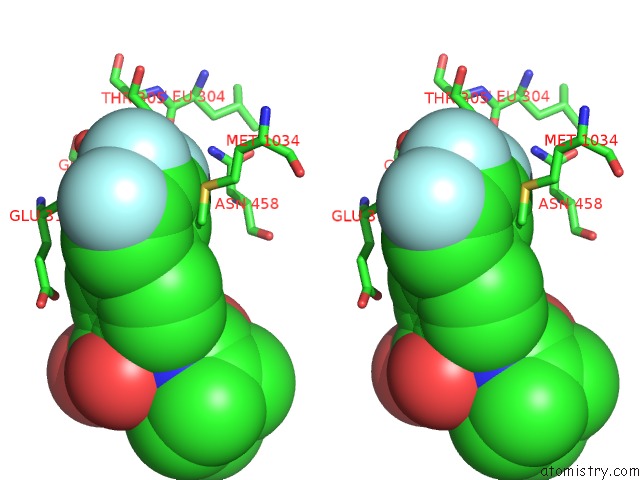
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6I) and Catalytic Zinc Ion within 5.0Å range:
|
Fluorine binding site 2 out of 3 in 6eaa
Go back to
Fluorine binding site 2 out
of 3 in the X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6I) and Catalytic Zinc Ion
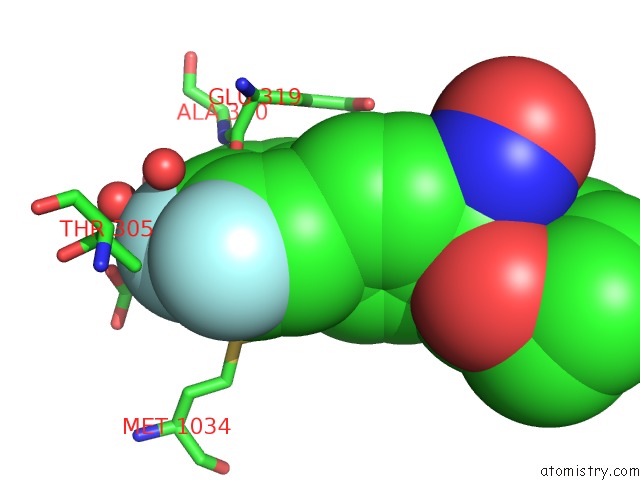
Mono view
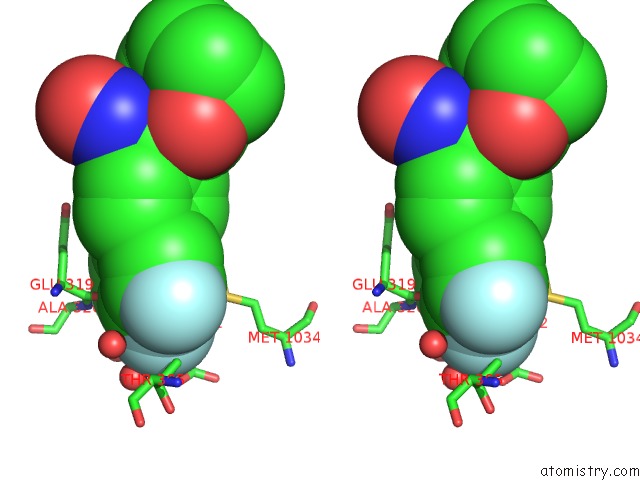
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6I) and Catalytic Zinc Ion within 5.0Å range:
|
Fluorine binding site 3 out of 3 in 6eaa
Go back to
Fluorine binding site 3 out
of 3 in the X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6I) and Catalytic Zinc Ion
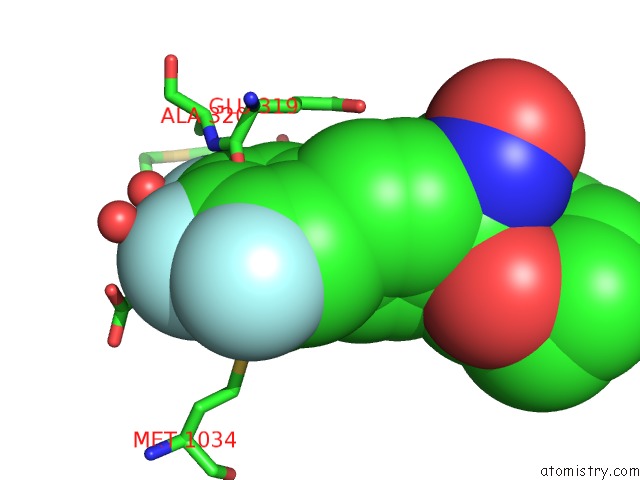
Mono view
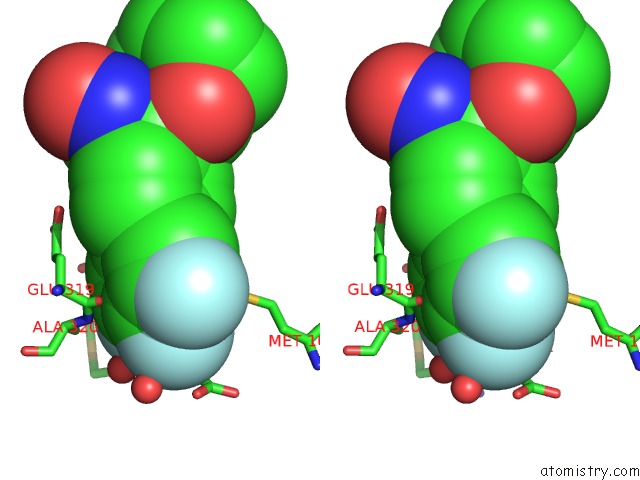
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of X-Ray Crystal Structure of Pf-M1 in Complex with Inhibitor (6I) and Catalytic Zinc Ion within 5.0Å range:
|
Reference:
N.B.Vinh,
N.Drinkwater,
T.R.Malcolm,
M.Kassiou,
L.Lucantoni,
P.M.Grin,
G.S.Butler,
S.Duffy,
C.M.Overall,
V.M.Avery,
P.J.Scammells,
S.Mcgowan.
Hydroxamic Acid Inhibitors Provide Cross-Species Inhibition of Plasmodium M1 and M17 Aminopeptidases. J. Med. Chem. V. 62 622 2019.
ISSN: ISSN 1520-4804
PubMed: 30537832
DOI: 10.1021/ACS.JMEDCHEM.8B01310
Page generated: Thu Aug 1 19:27:26 2024
ISSN: ISSN 1520-4804
PubMed: 30537832
DOI: 10.1021/ACS.JMEDCHEM.8B01310
Last articles
Cl in 5UZSCl in 5UZU
Cl in 5UZC
Cl in 5UZE
Cl in 5UZR
Cl in 5UZH
Cl in 5UZ8
Cl in 5UY1
Cl in 5UXZ
Cl in 5UXE