Fluorine »
PDB 6eog-6fet »
6f78 »
Fluorine in PDB 6f78: Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid
Enzymatic activity of Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid
All present enzymatic activity of Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid:
1.1.1.112; 1.1.1.188; 1.1.1.239; 1.1.1.357; 1.1.1.64;
1.1.1.112; 1.1.1.188; 1.1.1.239; 1.1.1.357; 1.1.1.64;
Protein crystallography data
The structure of Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid, PDB code: 6f78
was solved by
P.Goyal,
W.Y.Wahlgren,
R.Friemann,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 44.37 / 1.30 |
| Space group | P 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 47.203, 49.151, 83.386, 73.97, 86.68, 69.80 |
| R / Rfree (%) | 14.3 / 16.9 |
Other elements in 6f78:
The structure of Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid also contains other interesting chemical elements:
| Chlorine | (Cl) | 2 atoms |
Fluorine Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 12;Binding sites:
The binding sites of Fluorine atom in the Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid (pdb code 6f78). This binding sites where shown within 5.0 Angstroms radius around Fluorine atom.In total 12 binding sites of Fluorine where determined in the Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid, PDB code: 6f78:
Jump to Fluorine binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Fluorine binding site 1 out of 12 in 6f78
Go back to
Fluorine binding site 1 out
of 12 in the Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid
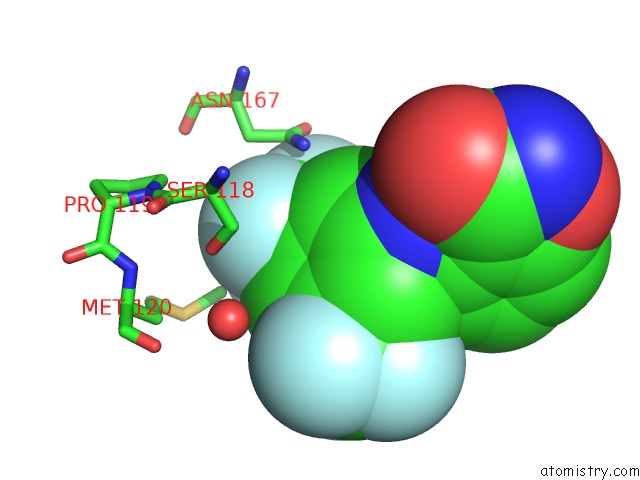
Mono view
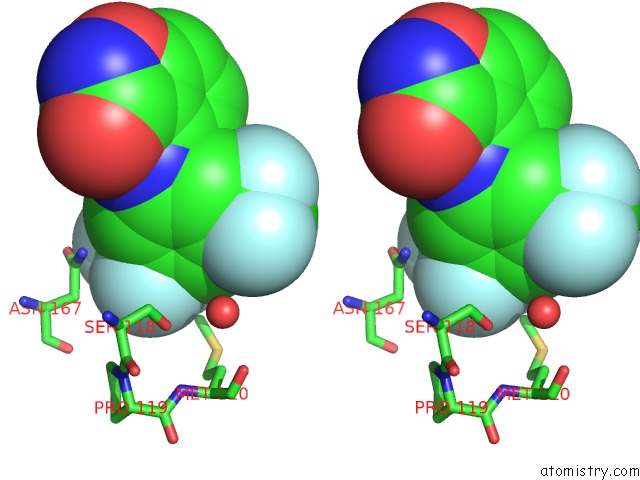
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid within 5.0Å range:
|
Fluorine binding site 2 out of 12 in 6f78
Go back to
Fluorine binding site 2 out
of 12 in the Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid
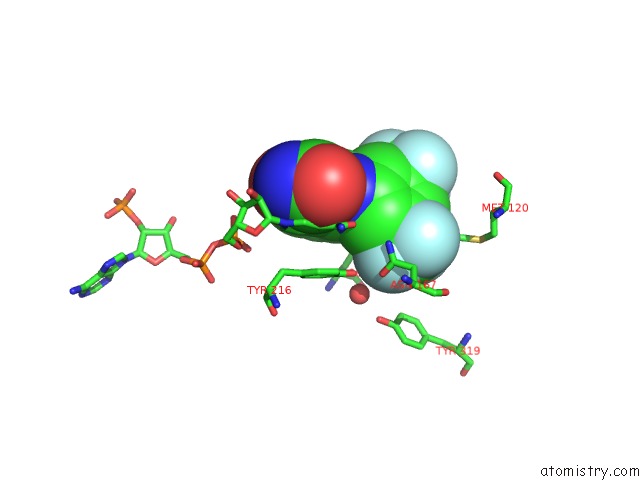
Mono view
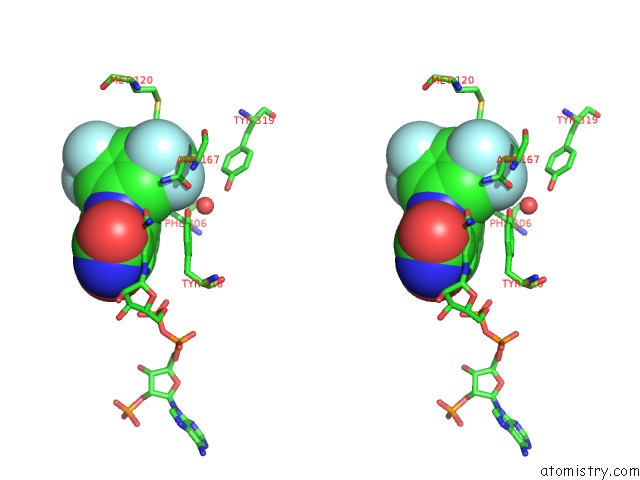
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid within 5.0Å range:
|
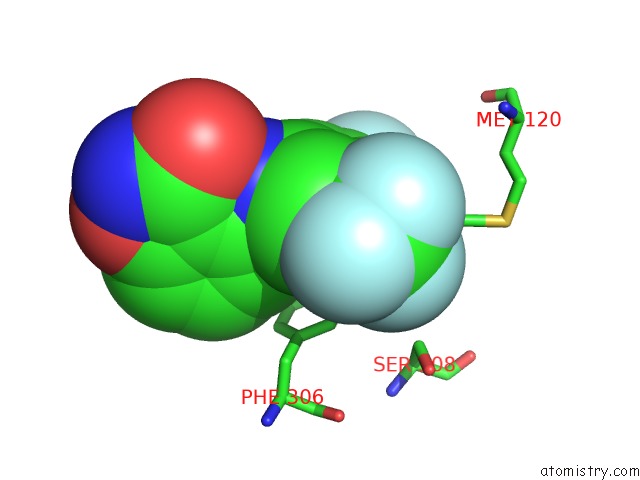
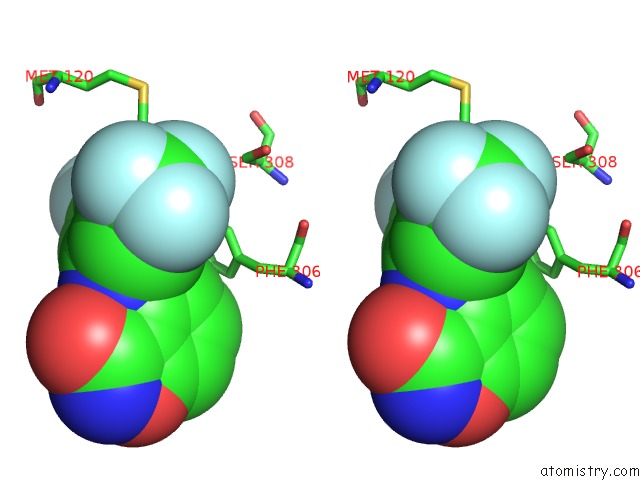
Fluorine binding site 3 out of 12 in 6f78
Go back to
Fluorine binding site 3 out
of 12 in the Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid within 5.0Å range:
|
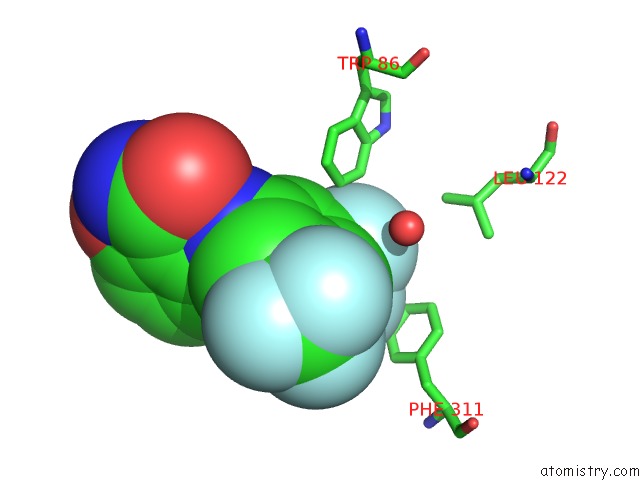
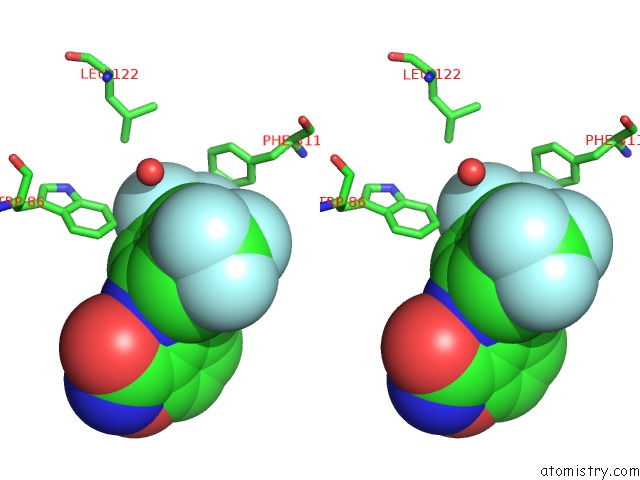
Fluorine binding site 4 out of 12 in 6f78
Go back to
Fluorine binding site 4 out
of 12 in the Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 4 of Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid within 5.0Å range:
|
Fluorine binding site 5 out of 12 in 6f78
Go back to
Fluorine binding site 5 out
of 12 in the Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid
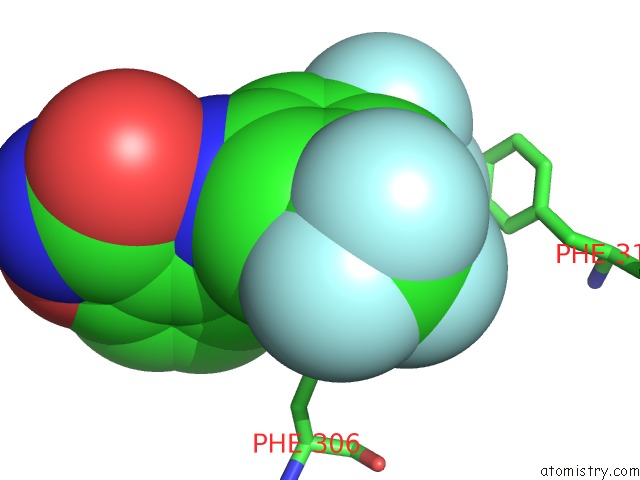
Mono view
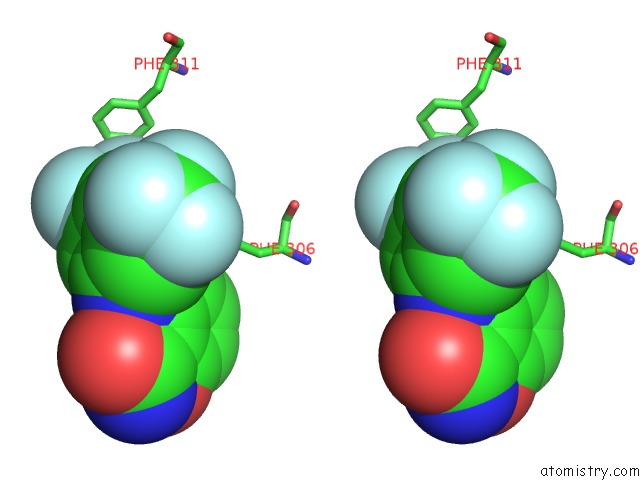
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 5 of Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid within 5.0Å range:
|
Fluorine binding site 6 out of 12 in 6f78
Go back to
Fluorine binding site 6 out
of 12 in the Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid
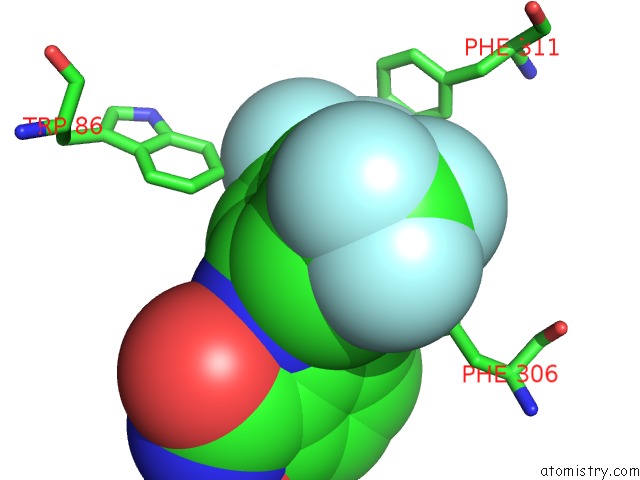
Mono view
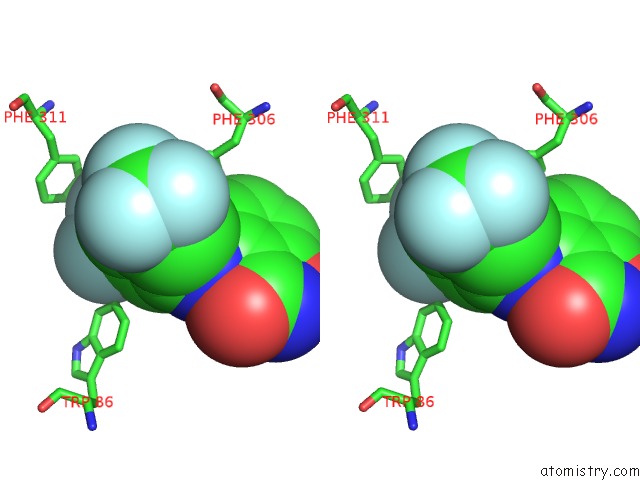
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 6 of Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid within 5.0Å range:
|
Fluorine binding site 7 out of 12 in 6f78
Go back to
Fluorine binding site 7 out
of 12 in the Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid
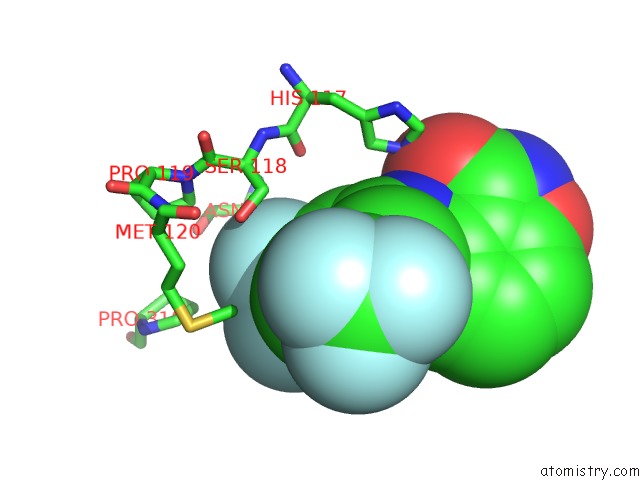
Mono view
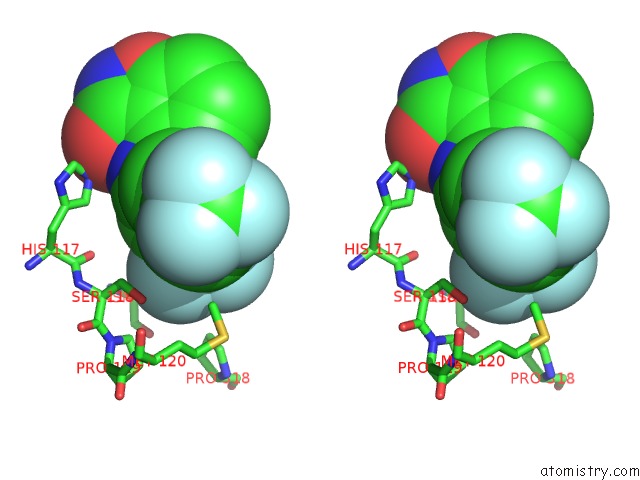
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 7 of Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid within 5.0Å range:
|
Fluorine binding site 8 out of 12 in 6f78
Go back to
Fluorine binding site 8 out
of 12 in the Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid
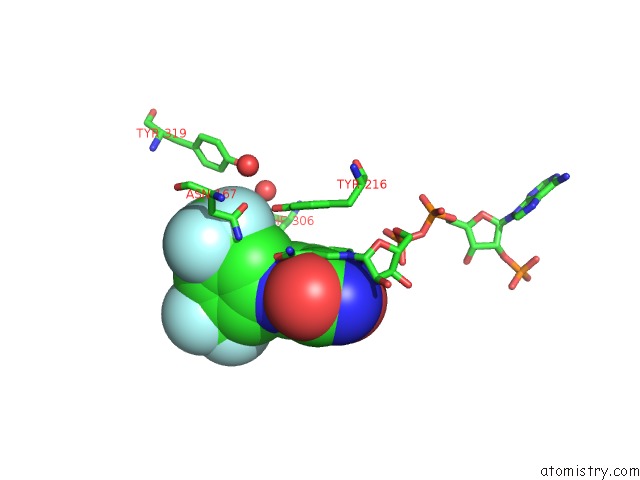
Mono view
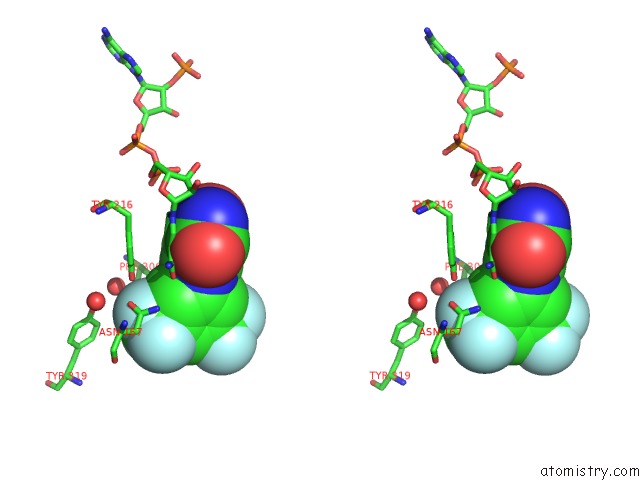
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 8 of Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid within 5.0Å range:
|
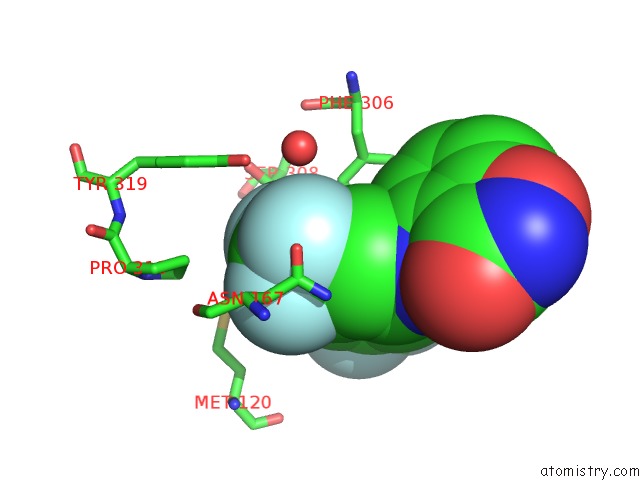
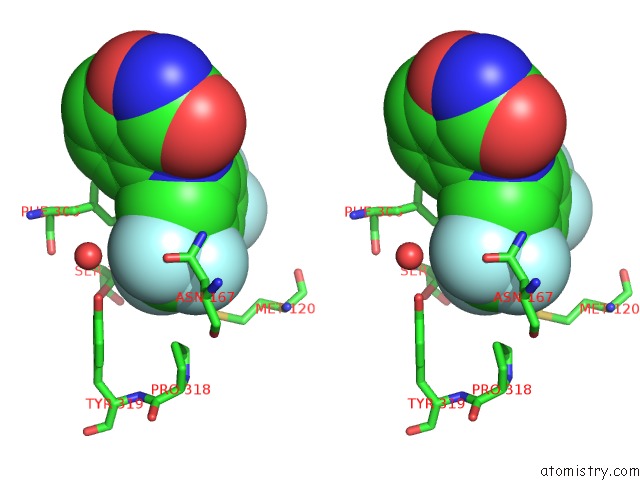
Fluorine binding site 9 out of 12 in 6f78
Go back to
Fluorine binding site 9 out
of 12 in the Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 9 of Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid within 5.0Å range:
|
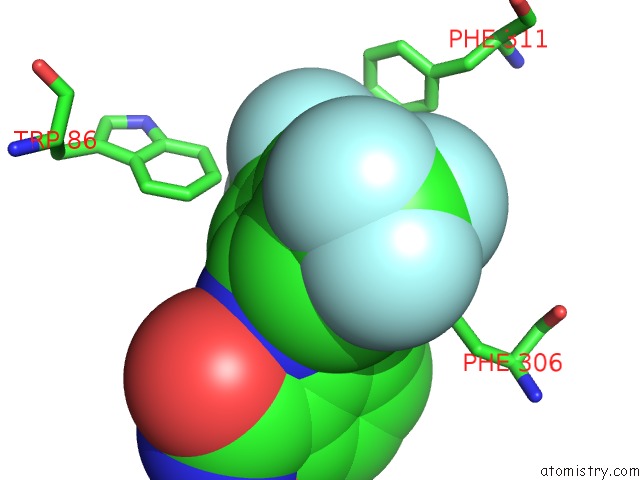
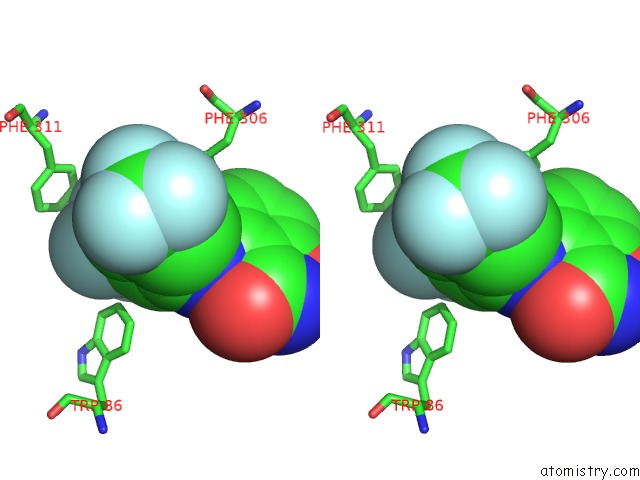
Fluorine binding site 10 out of 12 in 6f78
Go back to
Fluorine binding site 10 out
of 12 in the Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 10 of Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid within 5.0Å range:
|
Reference:
A.C.Pippione,
I.M.Carnovale,
D.Bonanni,
M.Sini,
P.Goyal,
E.Marini,
K.Pors,
S.Adinolfi,
D.Zonari,
C.Festuccia,
W.Y.Wahlgren,
R.Friemann,
R.Bagnati,
D.Boschi,
S.Oliaro-Bosso,
M.L.Lolli.
Potent and Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Based on the Benzoisoxazole Moiety: Application of A Bioisosteric Scaffold Hopping Approach to Flufenamic Acid. Eur J Med Chem V. 150 930 2018.
ISSN: ISSN 1768-3254
PubMed: 29602039
DOI: 10.1016/J.EJMECH.2018.03.040
Page generated: Thu Aug 1 19:52:13 2024
ISSN: ISSN 1768-3254
PubMed: 29602039
DOI: 10.1016/J.EJMECH.2018.03.040
Last articles
Ca in 5SSXCa in 5SV0
Ca in 5STD
Ca in 5SSZ
Ca in 5SSY
Ca in 5SIC
Ca in 5SBD
Ca in 5SBE
Ca in 5SBC
Ca in 5SBB