Fluorine »
PDB 6hmp-6i77 »
6hof »
Fluorine in PDB 6hof: Transcriptional Repressor Ethr From Mycobacterium Tuberculosis in Complex with BDM44852
Protein crystallography data
The structure of Transcriptional Repressor Ethr From Mycobacterium Tuberculosis in Complex with BDM44852, PDB code: 6hof
was solved by
R.Wintjens,
A.Wohlkonig,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 43.08 / 1.80 |
| Space group | P 41 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 121.849, 121.849, 33.766, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 17.9 / 20.9 |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Transcriptional Repressor Ethr From Mycobacterium Tuberculosis in Complex with BDM44852
(pdb code 6hof). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 6 binding sites of Fluorine where determined in the Transcriptional Repressor Ethr From Mycobacterium Tuberculosis in Complex with BDM44852, PDB code: 6hof:
Jump to Fluorine binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Fluorine where determined in the Transcriptional Repressor Ethr From Mycobacterium Tuberculosis in Complex with BDM44852, PDB code: 6hof:
Jump to Fluorine binding site number: 1; 2; 3; 4; 5; 6;
Fluorine binding site 1 out of 6 in 6hof
Go back to
Fluorine binding site 1 out
of 6 in the Transcriptional Repressor Ethr From Mycobacterium Tuberculosis in Complex with BDM44852

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Transcriptional Repressor Ethr From Mycobacterium Tuberculosis in Complex with BDM44852 within 5.0Å range:
|
Fluorine binding site 2 out of 6 in 6hof
Go back to
Fluorine binding site 2 out
of 6 in the Transcriptional Repressor Ethr From Mycobacterium Tuberculosis in Complex with BDM44852

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Transcriptional Repressor Ethr From Mycobacterium Tuberculosis in Complex with BDM44852 within 5.0Å range:
|
Fluorine binding site 3 out of 6 in 6hof
Go back to
Fluorine binding site 3 out
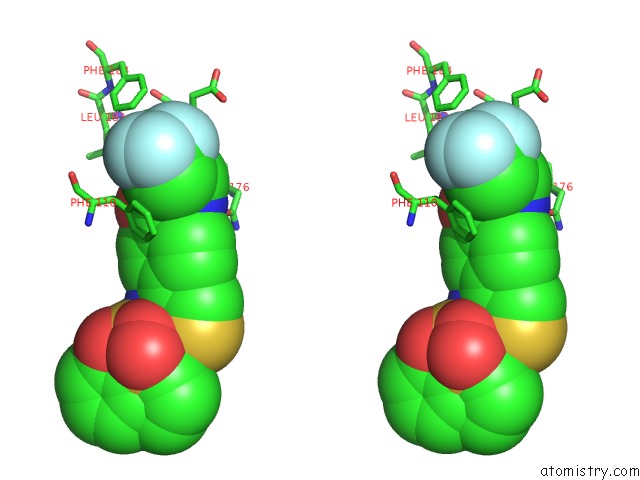
of 6 in the Transcriptional Repressor Ethr From Mycobacterium Tuberculosis in Complex with BDM44852

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Transcriptional Repressor Ethr From Mycobacterium Tuberculosis in Complex with BDM44852 within 5.0Å range:
|
Fluorine binding site 4 out of 6 in 6hof
Go back to
Fluorine binding site 4 out
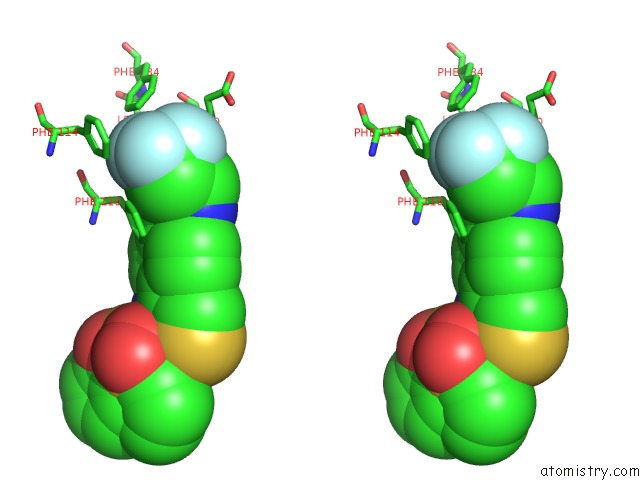
of 6 in the Transcriptional Repressor Ethr From Mycobacterium Tuberculosis in Complex with BDM44852

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 4 of Transcriptional Repressor Ethr From Mycobacterium Tuberculosis in Complex with BDM44852 within 5.0Å range:
|
Fluorine binding site 5 out of 6 in 6hof
Go back to
Fluorine binding site 5 out
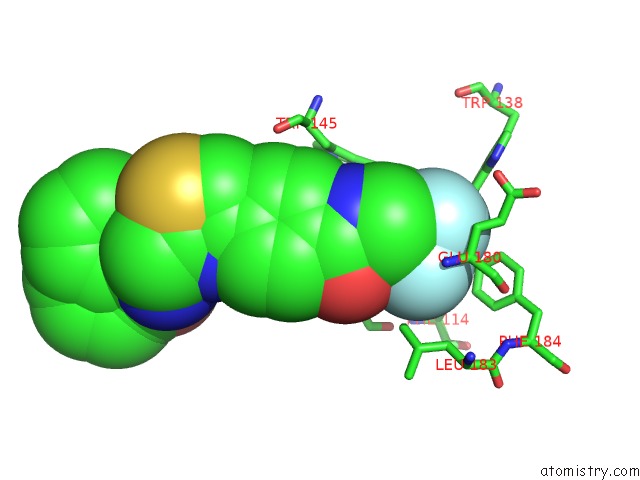
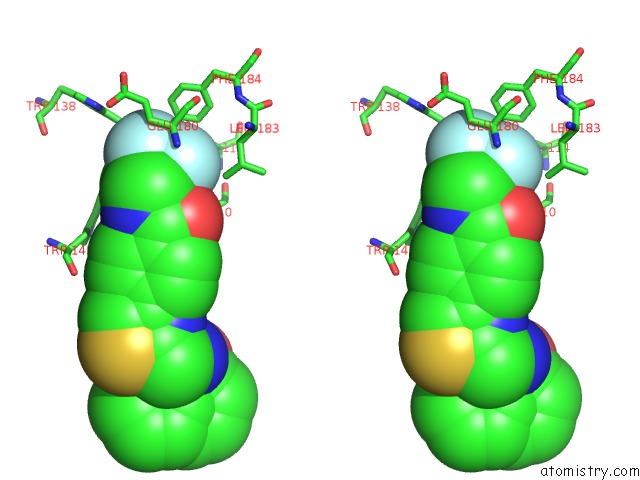
of 6 in the Transcriptional Repressor Ethr From Mycobacterium Tuberculosis in Complex with BDM44852
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 5 of Transcriptional Repressor Ethr From Mycobacterium Tuberculosis in Complex with BDM44852 within 5.0Å range:
|
Fluorine binding site 6 out of 6 in 6hof
Go back to
Fluorine binding site 6 out
of 6 in the Transcriptional Repressor Ethr From Mycobacterium Tuberculosis in Complex with BDM44852
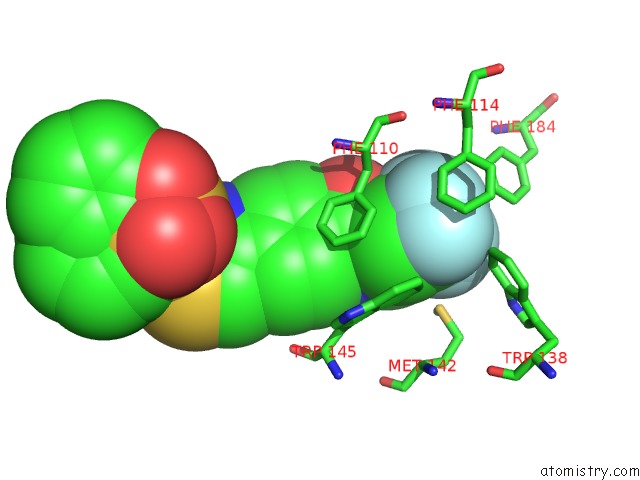
Mono view
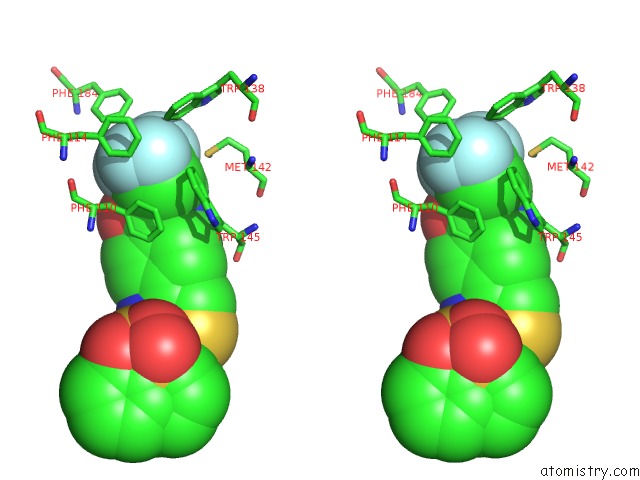
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 6 of Transcriptional Repressor Ethr From Mycobacterium Tuberculosis in Complex with BDM44852 within 5.0Å range:
|
Reference:
A.Tanina,
A.Wohlkonig,
S.H.Soror,
M.Flipo,
B.Villemagne,
H.Prevet,
B.Deprez,
M.Moune,
H.Peree,
F.Meyer,
A.R.Baulard,
N.Willand,
R.Wintjens.
A Comprehensive Analysis of the Protein-Ligand Interactions in Crystal Structures of Mycobacterium Tuberculosis Ethr. Biochim Biophys Acta V.1867 248 2018PROTEINS Proteom.
ISSN: ISSN 1878-1454
PubMed: 30553830
DOI: 10.1016/J.BBAPAP.2018.12.003
Page generated: Thu Aug 1 21:17:24 2024
ISSN: ISSN 1878-1454
PubMed: 30553830
DOI: 10.1016/J.BBAPAP.2018.12.003
Last articles
Cl in 3DOLCl in 3DOK
Cl in 3DOJ
Cl in 3DNU
Cl in 3DNA
Cl in 3DN1
Cl in 3DN6
Cl in 3DN8
Cl in 3DN0
Cl in 3DMZ