Fluorine »
PDB 6q13-6qhw »
6q7w »
Fluorine in PDB 6q7w: Crystal Structure of Pqsr (Mvfr) Ligand-Binding Domain in Complex with Compound 20
Protein crystallography data
The structure of Crystal Structure of Pqsr (Mvfr) Ligand-Binding Domain in Complex with Compound 20, PDB code: 6q7w
was solved by
F.Witzgall,
W.Blankenfeldt,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 47.72 / 2.82 |
| Space group | P 65 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 121.218, 121.218, 114.555, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 23.1 / 26.2 |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Crystal Structure of Pqsr (Mvfr) Ligand-Binding Domain in Complex with Compound 20
(pdb code 6q7w). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 4 binding sites of Fluorine where determined in the Crystal Structure of Pqsr (Mvfr) Ligand-Binding Domain in Complex with Compound 20, PDB code: 6q7w:
Jump to Fluorine binding site number: 1; 2; 3; 4;
In total 4 binding sites of Fluorine where determined in the Crystal Structure of Pqsr (Mvfr) Ligand-Binding Domain in Complex with Compound 20, PDB code: 6q7w:
Jump to Fluorine binding site number: 1; 2; 3; 4;
Fluorine binding site 1 out of 4 in 6q7w
Go back to
Fluorine binding site 1 out
of 4 in the Crystal Structure of Pqsr (Mvfr) Ligand-Binding Domain in Complex with Compound 20
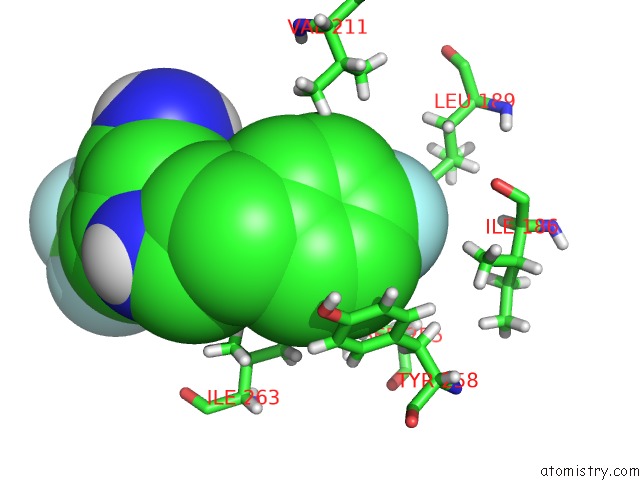
Mono view
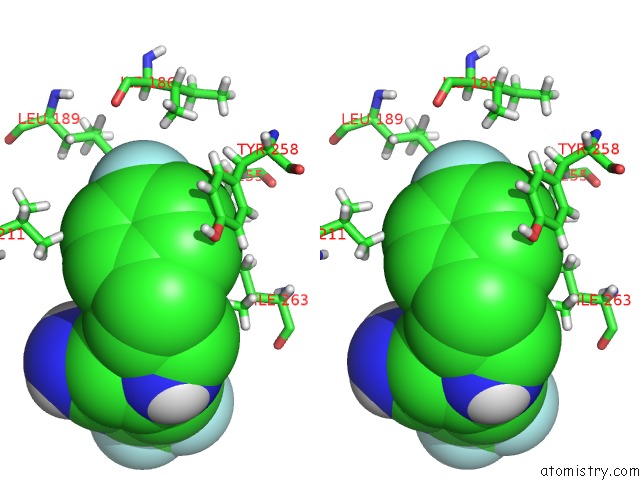
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Crystal Structure of Pqsr (Mvfr) Ligand-Binding Domain in Complex with Compound 20 within 5.0Å range:
|
Fluorine binding site 2 out of 4 in 6q7w
Go back to
Fluorine binding site 2 out
of 4 in the Crystal Structure of Pqsr (Mvfr) Ligand-Binding Domain in Complex with Compound 20
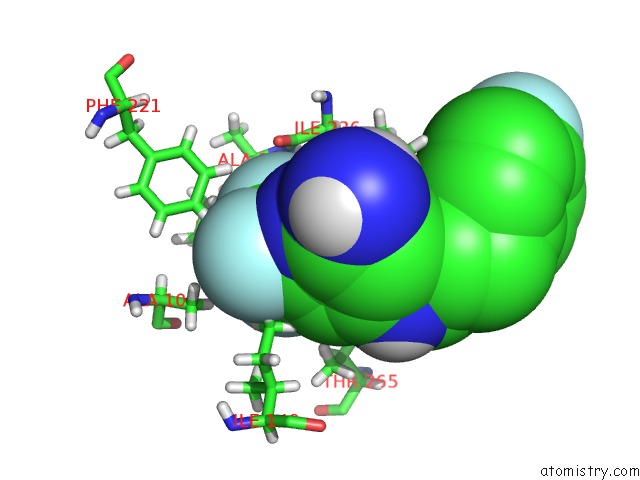
Mono view
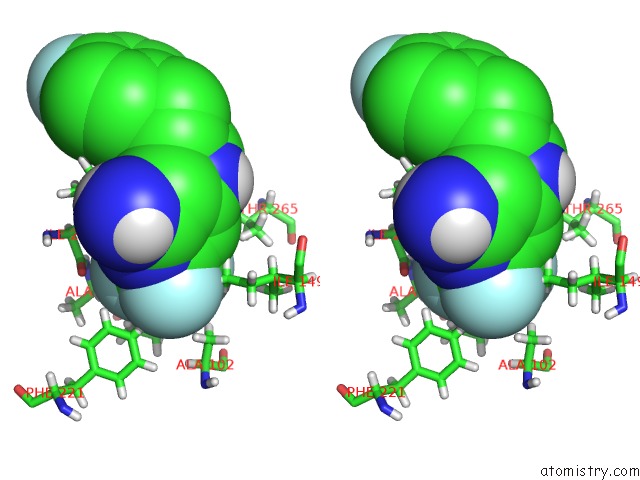
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Crystal Structure of Pqsr (Mvfr) Ligand-Binding Domain in Complex with Compound 20 within 5.0Å range:
|
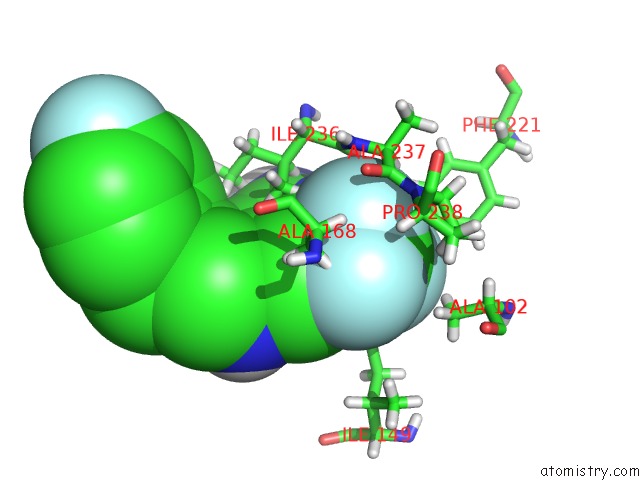
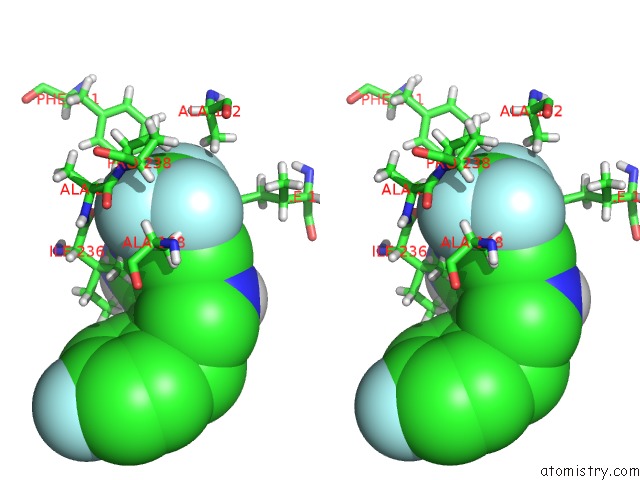
Fluorine binding site 3 out of 4 in 6q7w
Go back to
Fluorine binding site 3 out
of 4 in the Crystal Structure of Pqsr (Mvfr) Ligand-Binding Domain in Complex with Compound 20
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Crystal Structure of Pqsr (Mvfr) Ligand-Binding Domain in Complex with Compound 20 within 5.0Å range:
|
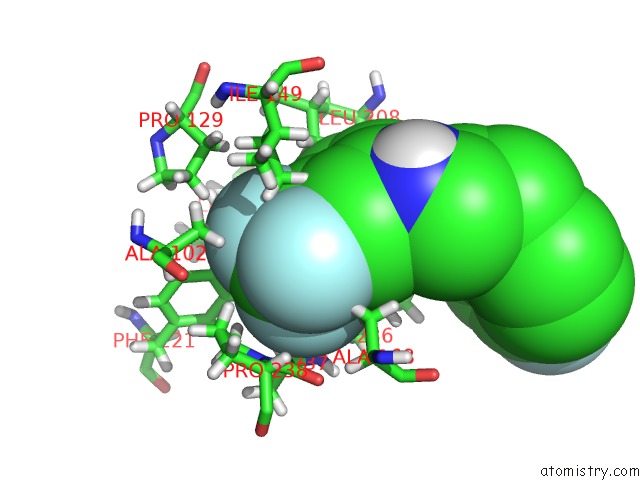
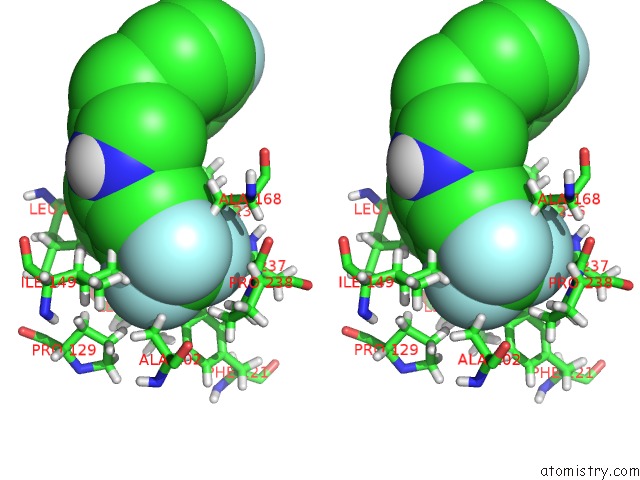
Fluorine binding site 4 out of 4 in 6q7w
Go back to
Fluorine binding site 4 out
of 4 in the Crystal Structure of Pqsr (Mvfr) Ligand-Binding Domain in Complex with Compound 20
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 4 of Crystal Structure of Pqsr (Mvfr) Ligand-Binding Domain in Complex with Compound 20 within 5.0Å range:
|
Reference:
M.Zender,
F.Witzgall,
A.F.Kiefer,
B.Kirsch,
C.K.Maurer,
A.M.Kany,
N.Xu,
S.Schmelz,
C.Borger,
W.Blankenfeldt,
M.Empting.
Flexible Fragment Growing Boosts Potency of Quorum Sensing Inhibitors Against Pseudomonas Aeruginosa Virulence. Chemmedchem 2019.
ISSN: ESSN 1860-7187
PubMed: 31709767
DOI: 10.1002/CMDC.201900621
Page generated: Tue Jul 15 14:58:10 2025
ISSN: ESSN 1860-7187
PubMed: 31709767
DOI: 10.1002/CMDC.201900621
Last articles
F in 7LY8F in 7LWG
F in 7LZV
F in 7LZF
F in 7LZD
F in 7LZA
F in 7LVX
F in 7LUN
F in 7LVR
F in 7LUK