Fluorine »
PDB 6rkp-6rzg »
6roi »
Fluorine in PDB 6roi: Cryo-Em Structure of the Partially Activated DRS2P-CDC50P
Enzymatic activity of Cryo-Em Structure of the Partially Activated DRS2P-CDC50P
All present enzymatic activity of Cryo-Em Structure of the Partially Activated DRS2P-CDC50P:
7.6.2.1;
7.6.2.1;
Other elements in 6roi:
The structure of Cryo-Em Structure of the Partially Activated DRS2P-CDC50P also contains other interesting chemical elements:
| Magnesium | (Mg) | 1 atom |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Cryo-Em Structure of the Partially Activated DRS2P-CDC50P
(pdb code 6roi). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 3 binding sites of Fluorine where determined in the Cryo-Em Structure of the Partially Activated DRS2P-CDC50P, PDB code: 6roi:
Jump to Fluorine binding site number: 1; 2; 3;
In total 3 binding sites of Fluorine where determined in the Cryo-Em Structure of the Partially Activated DRS2P-CDC50P, PDB code: 6roi:
Jump to Fluorine binding site number: 1; 2; 3;
Fluorine binding site 1 out of 3 in 6roi
Go back to
Fluorine binding site 1 out
of 3 in the Cryo-Em Structure of the Partially Activated DRS2P-CDC50P
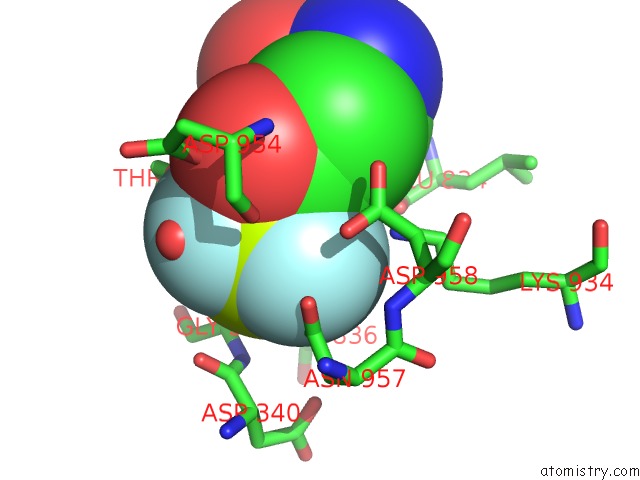
Mono view
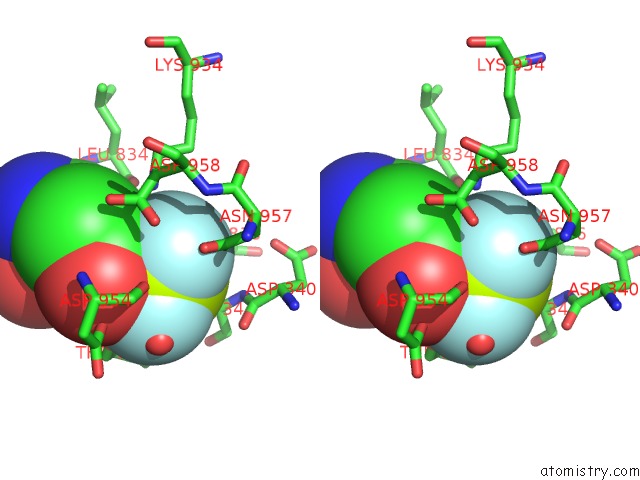
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Cryo-Em Structure of the Partially Activated DRS2P-CDC50P within 5.0Å range:
|
Fluorine binding site 2 out of 3 in 6roi
Go back to
Fluorine binding site 2 out
of 3 in the Cryo-Em Structure of the Partially Activated DRS2P-CDC50P
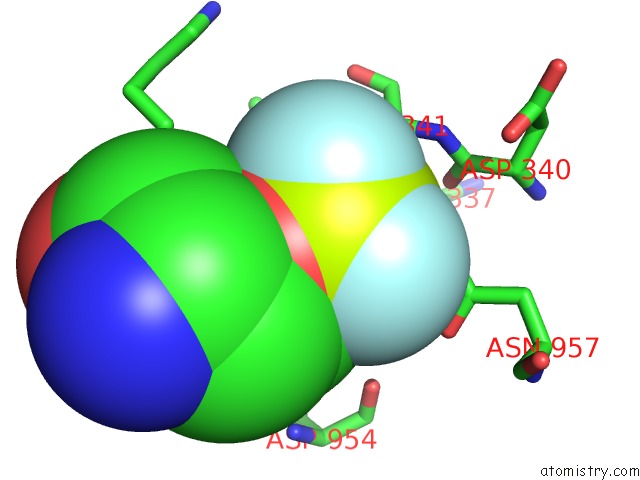
Mono view
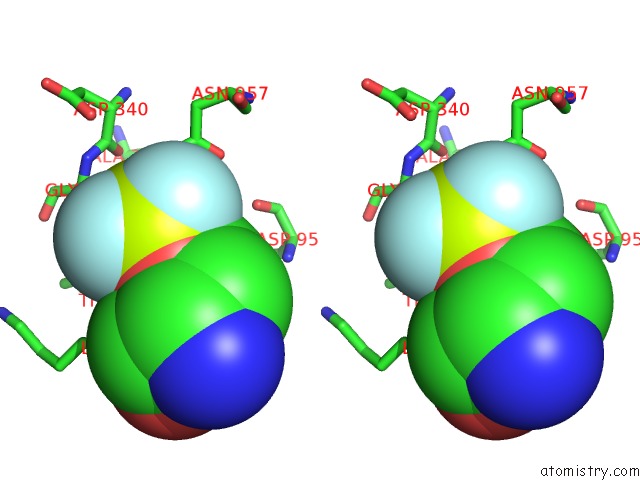
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Cryo-Em Structure of the Partially Activated DRS2P-CDC50P within 5.0Å range:
|
Fluorine binding site 3 out of 3 in 6roi
Go back to
Fluorine binding site 3 out
of 3 in the Cryo-Em Structure of the Partially Activated DRS2P-CDC50P
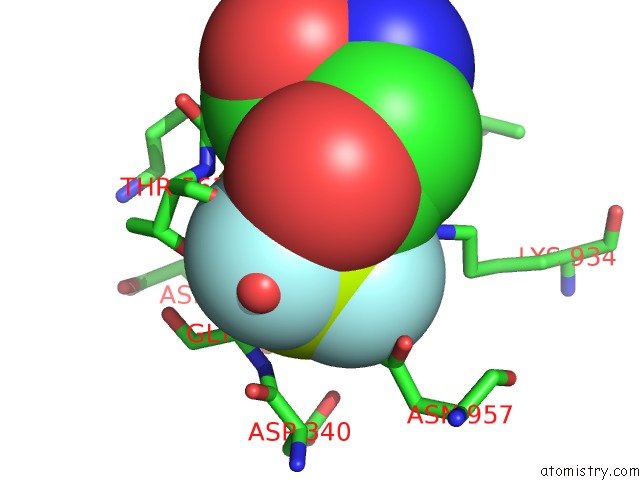
Mono view
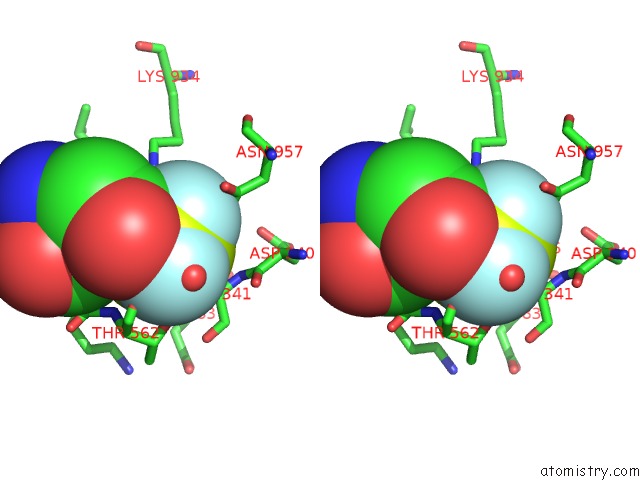
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Cryo-Em Structure of the Partially Activated DRS2P-CDC50P within 5.0Å range:
|
Reference:
M.Timcenko,
J.A.Lyons,
D.Januliene,
J.J.Ulstrup,
T.Dieudonne,
C.Montigny,
M.R.Ash,
J.L.Karlsen,
T.Boesen,
W.Kuhlbrandt,
G.Lenoir,
A.Moeller,
P.Nissen.
Structure and Autoregulation of A P4-Atpase Lipid Flippase. Nature V. 571 366 2019.
ISSN: ESSN 1476-4687
PubMed: 31243363
DOI: 10.1038/S41586-019-1344-7
Page generated: Fri Aug 2 01:19:04 2024
ISSN: ESSN 1476-4687
PubMed: 31243363
DOI: 10.1038/S41586-019-1344-7
Last articles
Zn in 9J0NZn in 9J0O
Zn in 9J0P
Zn in 9FJX
Zn in 9EKB
Zn in 9C0F
Zn in 9CAH
Zn in 9CH0
Zn in 9CH3
Zn in 9CH1