Fluorine »
PDB 6rzh-6slg »
6s4t »
Fluorine in PDB 6s4t: Lxrbeta Ligand Binding Domain in Comlpex with Small Molecule Inhibitors
Protein crystallography data
The structure of Lxrbeta Ligand Binding Domain in Comlpex with Small Molecule Inhibitors, PDB code: 6s4t
was solved by
J.Sandmark,
A.Jansson,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 62.02 / 2.00 |
| Space group | I 41 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 135.275, 135.275, 69.734, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 20.5 / 23.8 |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Lxrbeta Ligand Binding Domain in Comlpex with Small Molecule Inhibitors
(pdb code 6s4t). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 6 binding sites of Fluorine where determined in the Lxrbeta Ligand Binding Domain in Comlpex with Small Molecule Inhibitors, PDB code: 6s4t:
Jump to Fluorine binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Fluorine where determined in the Lxrbeta Ligand Binding Domain in Comlpex with Small Molecule Inhibitors, PDB code: 6s4t:
Jump to Fluorine binding site number: 1; 2; 3; 4; 5; 6;
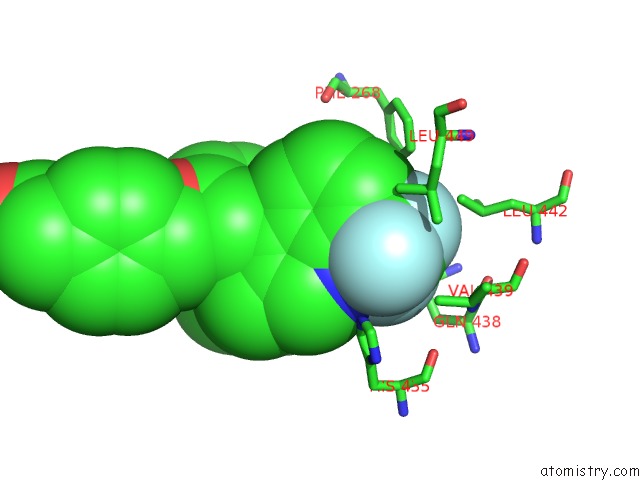
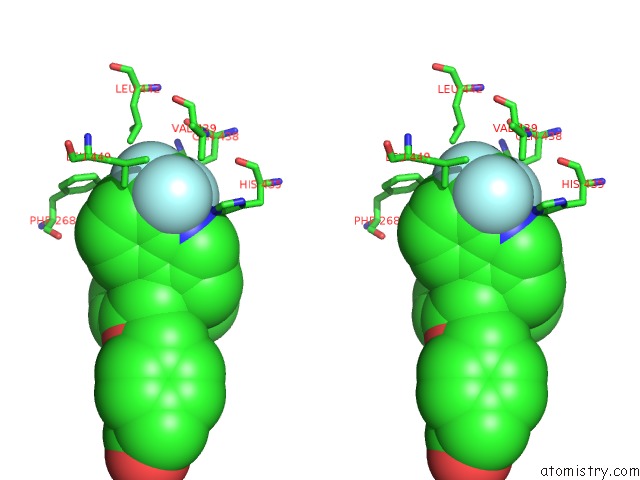
Fluorine binding site 1 out of 6 in 6s4t
Go back to
Fluorine binding site 1 out
of 6 in the Lxrbeta Ligand Binding Domain in Comlpex with Small Molecule Inhibitors
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Lxrbeta Ligand Binding Domain in Comlpex with Small Molecule Inhibitors within 5.0Å range:
|
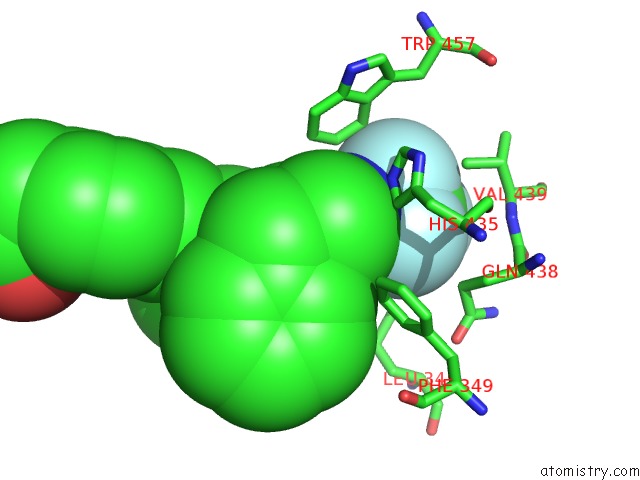
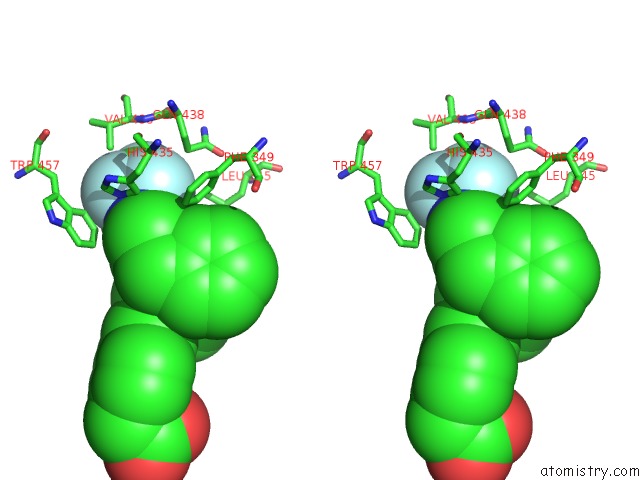
Fluorine binding site 2 out of 6 in 6s4t
Go back to
Fluorine binding site 2 out
of 6 in the Lxrbeta Ligand Binding Domain in Comlpex with Small Molecule Inhibitors
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Lxrbeta Ligand Binding Domain in Comlpex with Small Molecule Inhibitors within 5.0Å range:
|
Fluorine binding site 3 out of 6 in 6s4t
Go back to
Fluorine binding site 3 out
of 6 in the Lxrbeta Ligand Binding Domain in Comlpex with Small Molecule Inhibitors
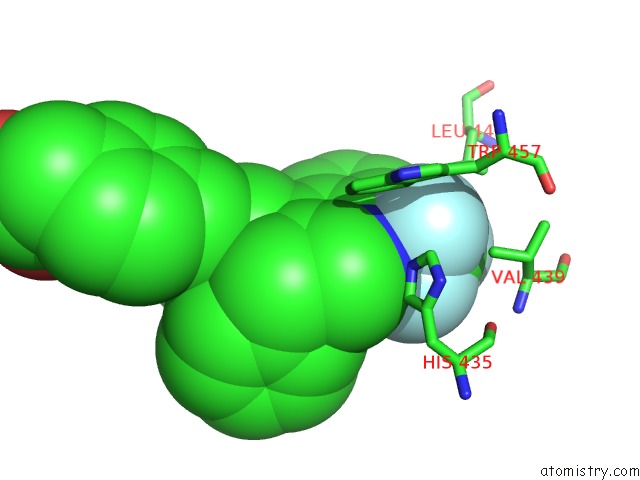
Mono view
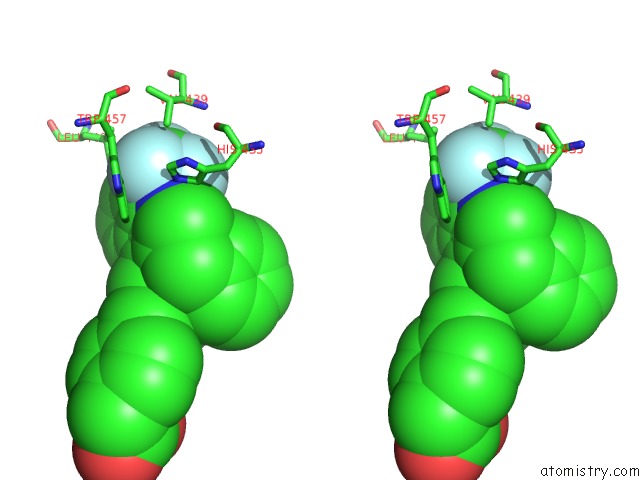
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Lxrbeta Ligand Binding Domain in Comlpex with Small Molecule Inhibitors within 5.0Å range:
|
Fluorine binding site 4 out of 6 in 6s4t
Go back to
Fluorine binding site 4 out
of 6 in the Lxrbeta Ligand Binding Domain in Comlpex with Small Molecule Inhibitors
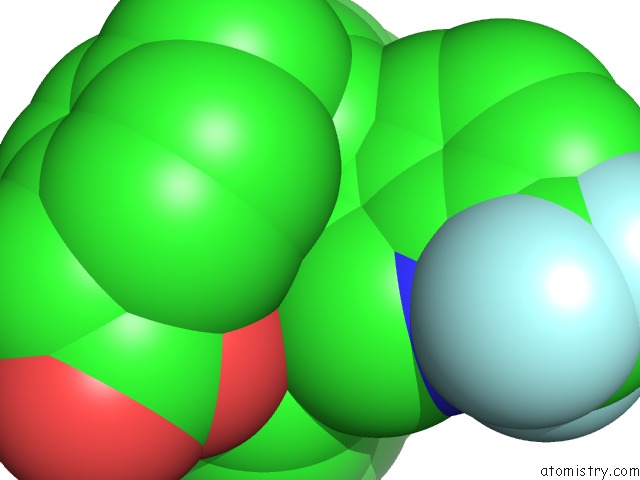
Mono view
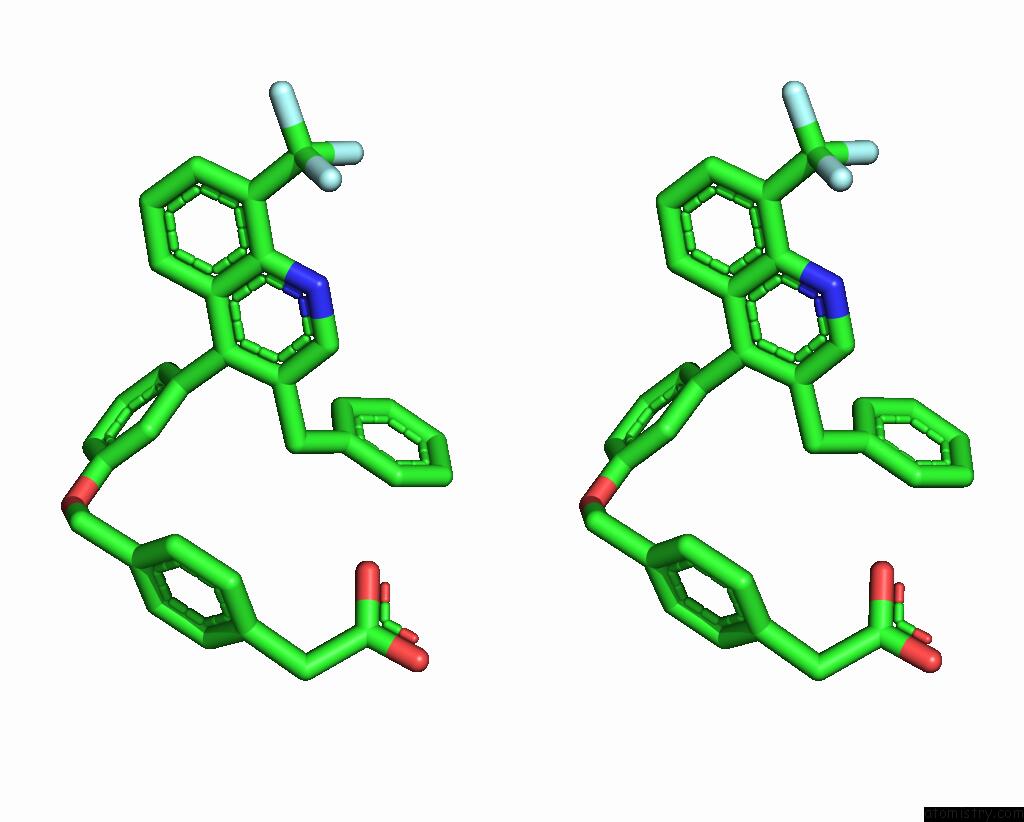
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 4 of Lxrbeta Ligand Binding Domain in Comlpex with Small Molecule Inhibitors within 5.0Å range:
|
Fluorine binding site 5 out of 6 in 6s4t
Go back to
Fluorine binding site 5 out
of 6 in the Lxrbeta Ligand Binding Domain in Comlpex with Small Molecule Inhibitors
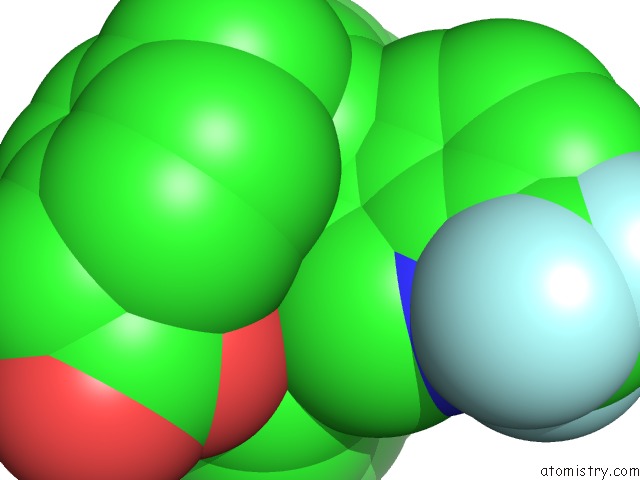
Mono view
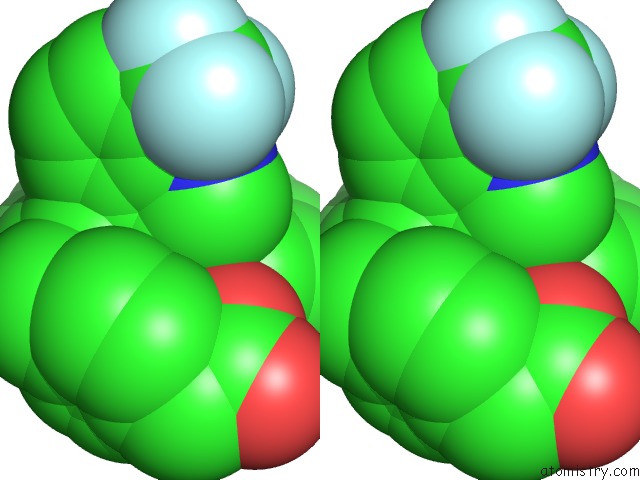
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 5 of Lxrbeta Ligand Binding Domain in Comlpex with Small Molecule Inhibitors within 5.0Å range:
|
Fluorine binding site 6 out of 6 in 6s4t
Go back to
Fluorine binding site 6 out
of 6 in the Lxrbeta Ligand Binding Domain in Comlpex with Small Molecule Inhibitors
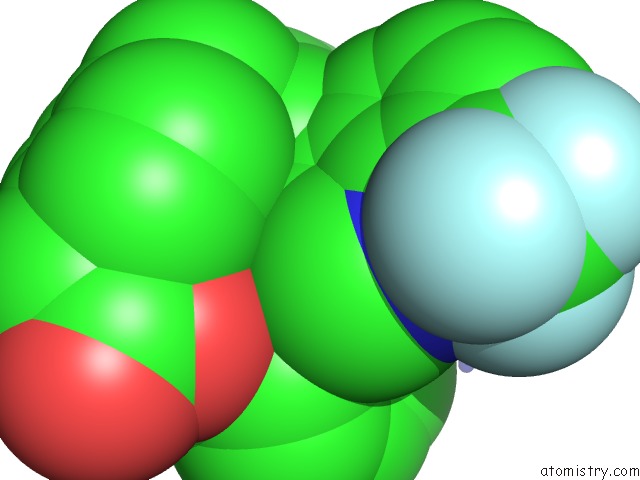
Mono view
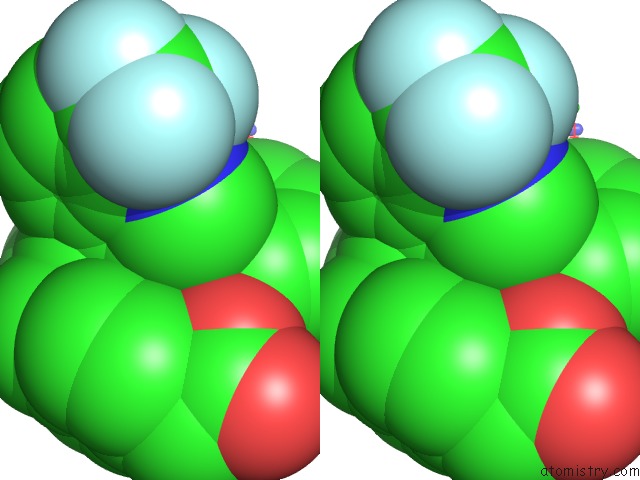
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 6 of Lxrbeta Ligand Binding Domain in Comlpex with Small Molecule Inhibitors within 5.0Å range:
|
Reference:
A.Y.Belorusova,
E.Evertsson,
D.Hovdal,
J.Sandmark,
E.Bratt,
I.Maxvall,
I.G.Schulman,
P.Akerblad,
E.Lindstedt.
Structural Analysis Identifies An Escape Route From the Adverse Lipogenic Effects of Liver X Receptor Ligands Commun Biol 2019.
ISSN: ESSN 2399-3642
DOI: 10.1038/S42003-019-0675-0
Page generated: Fri Aug 2 01:37:05 2024
ISSN: ESSN 2399-3642
DOI: 10.1038/S42003-019-0675-0
Last articles
Ca in 3DE0Ca in 3DDZ
Ca in 3DBZ
Ca in 3D4G
Ca in 3DC0
Ca in 3DB7
Ca in 3DAS
Ca in 3DBK
Ca in 3DAW
Ca in 3D9Q