Fluorine »
PDB 7db8-7e5h »
7drx »
Fluorine in PDB 7drx: Cryo-Em Structure of DNF1 From Saccharomyces Cerevisiae in 90PS with Beryllium Fluoride (E2P State)
Enzymatic activity of Cryo-Em Structure of DNF1 From Saccharomyces Cerevisiae in 90PS with Beryllium Fluoride (E2P State)
All present enzymatic activity of Cryo-Em Structure of DNF1 From Saccharomyces Cerevisiae in 90PS with Beryllium Fluoride (E2P State):
7.6.2.1;
7.6.2.1;
Other elements in 7drx:
The structure of Cryo-Em Structure of DNF1 From Saccharomyces Cerevisiae in 90PS with Beryllium Fluoride (E2P State) also contains other interesting chemical elements:
| Magnesium | (Mg) | 1 atom |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Cryo-Em Structure of DNF1 From Saccharomyces Cerevisiae in 90PS with Beryllium Fluoride (E2P State)
(pdb code 7drx). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 3 binding sites of Fluorine where determined in the Cryo-Em Structure of DNF1 From Saccharomyces Cerevisiae in 90PS with Beryllium Fluoride (E2P State), PDB code: 7drx:
Jump to Fluorine binding site number: 1; 2; 3;
In total 3 binding sites of Fluorine where determined in the Cryo-Em Structure of DNF1 From Saccharomyces Cerevisiae in 90PS with Beryllium Fluoride (E2P State), PDB code: 7drx:
Jump to Fluorine binding site number: 1; 2; 3;
Fluorine binding site 1 out of 3 in 7drx
Go back to
Fluorine binding site 1 out
of 3 in the Cryo-Em Structure of DNF1 From Saccharomyces Cerevisiae in 90PS with Beryllium Fluoride (E2P State)
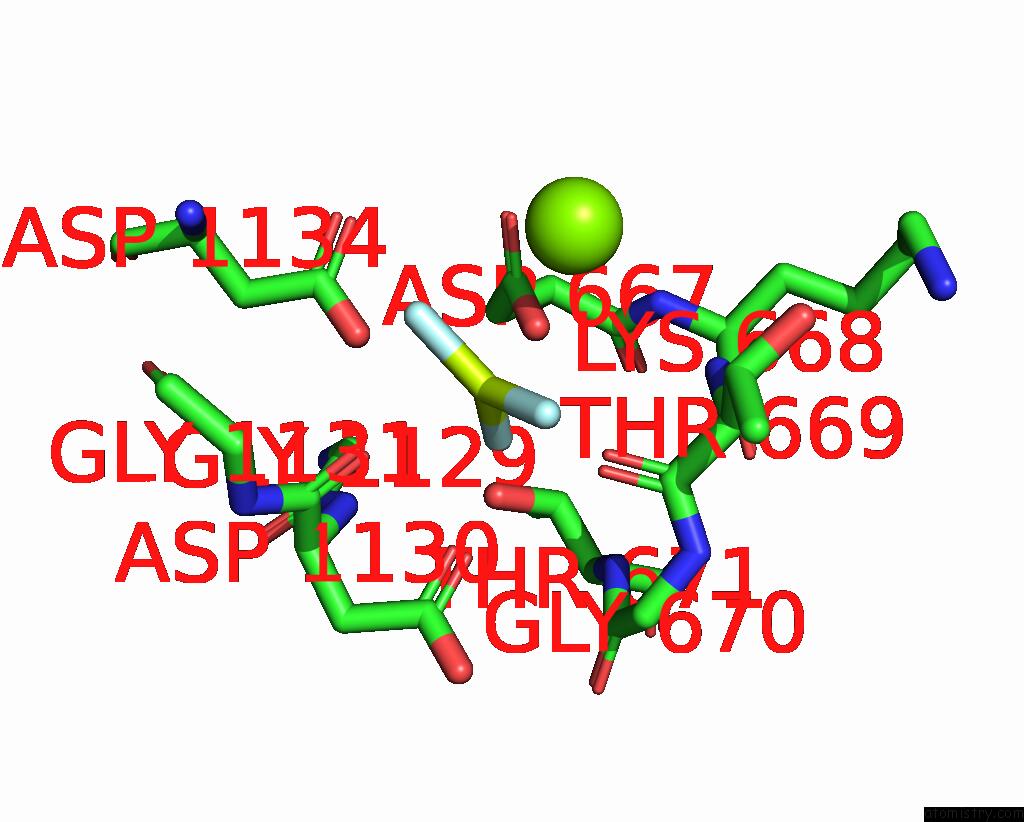
Mono view
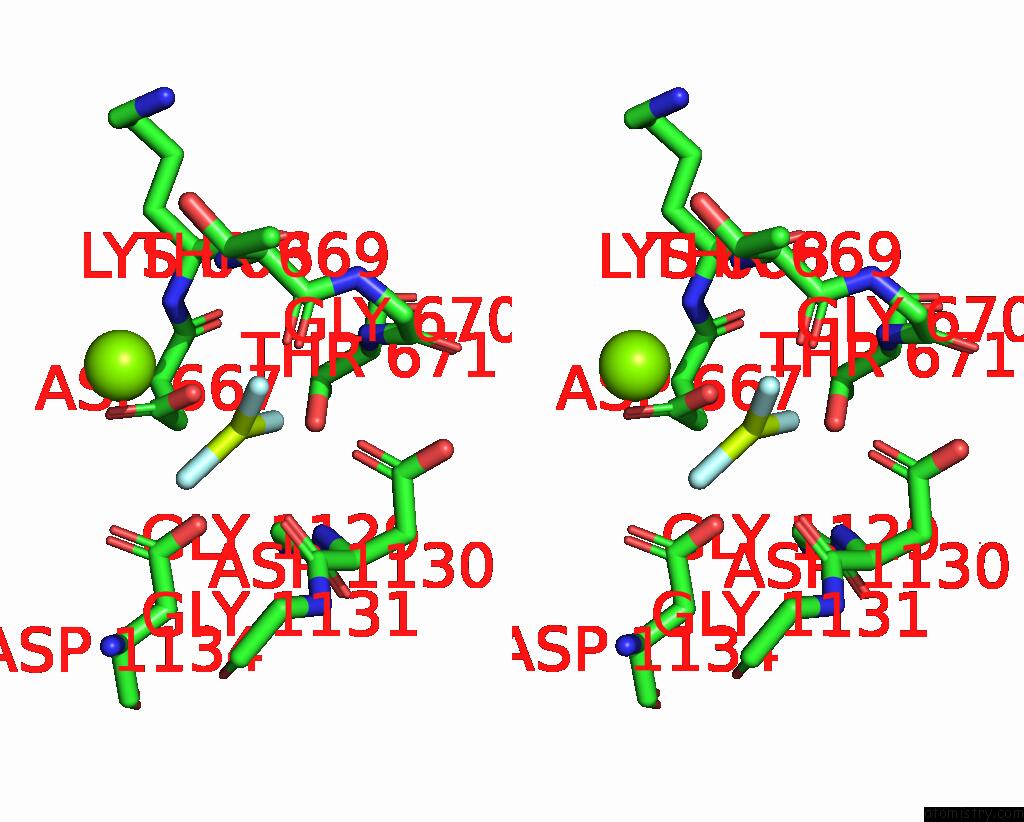
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Cryo-Em Structure of DNF1 From Saccharomyces Cerevisiae in 90PS with Beryllium Fluoride (E2P State) within 5.0Å range:
|
Fluorine binding site 2 out of 3 in 7drx
Go back to
Fluorine binding site 2 out
of 3 in the Cryo-Em Structure of DNF1 From Saccharomyces Cerevisiae in 90PS with Beryllium Fluoride (E2P State)
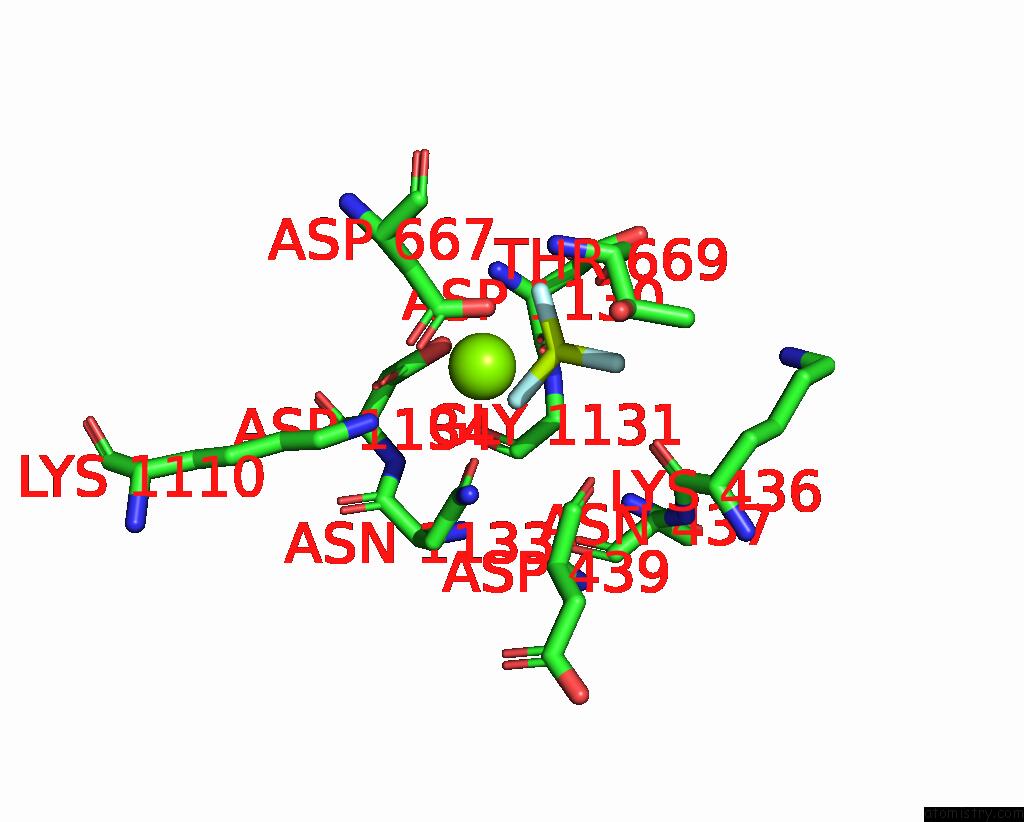
Mono view
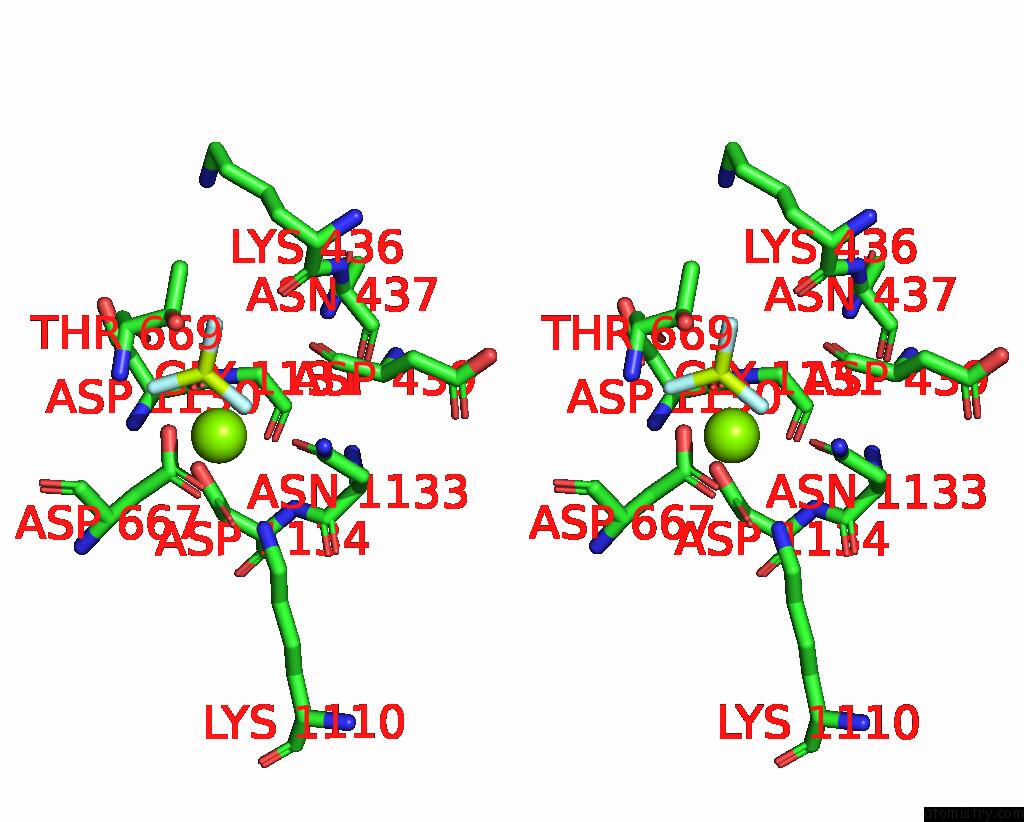
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Cryo-Em Structure of DNF1 From Saccharomyces Cerevisiae in 90PS with Beryllium Fluoride (E2P State) within 5.0Å range:
|
Fluorine binding site 3 out of 3 in 7drx
Go back to
Fluorine binding site 3 out
of 3 in the Cryo-Em Structure of DNF1 From Saccharomyces Cerevisiae in 90PS with Beryllium Fluoride (E2P State)
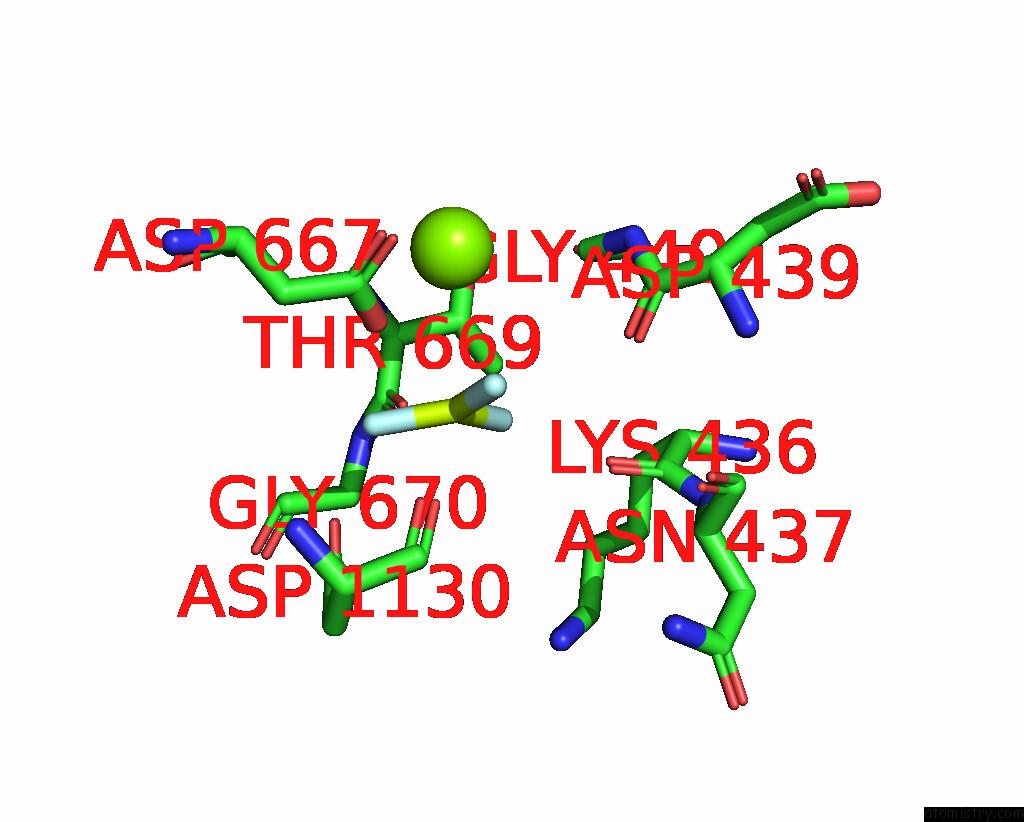
Mono view
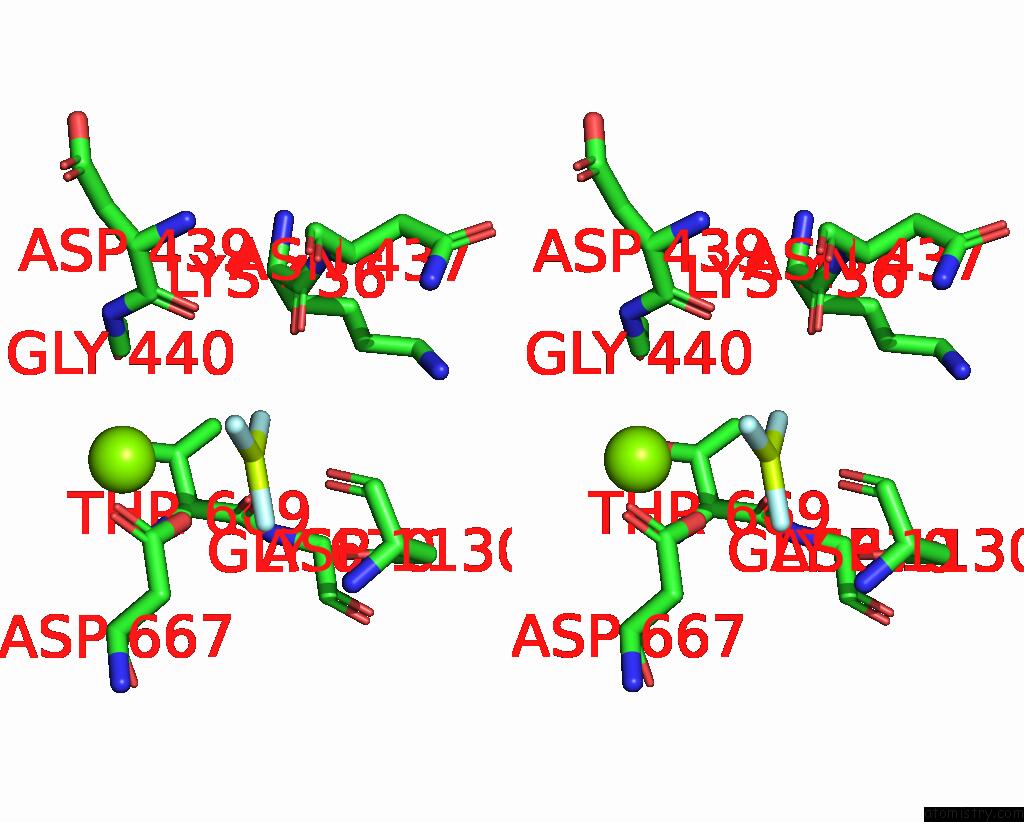
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Cryo-Em Structure of DNF1 From Saccharomyces Cerevisiae in 90PS with Beryllium Fluoride (E2P State) within 5.0Å range:
|
Reference:
J.Xu,
Y.He,
X.Wu,
L.Li.
Conformational Changes of A Phosphatidylcholine Flippase in Lipid Membranes. Cell Rep V. 38 10518 2022.
ISSN: ESSN 2211-1247
PubMed: 35294892
DOI: 10.1016/J.CELREP.2022.110518
Page generated: Fri Aug 2 06:29:30 2024
ISSN: ESSN 2211-1247
PubMed: 35294892
DOI: 10.1016/J.CELREP.2022.110518
Last articles
Zn in 9J0NZn in 9J0O
Zn in 9J0P
Zn in 9FJX
Zn in 9EKB
Zn in 9C0F
Zn in 9CAH
Zn in 9CH0
Zn in 9CH3
Zn in 9CH1