Fluorine »
PDB 5avz-5btd »
5aw2 »
Fluorine in PDB 5aw2: Kinetics By X-Ray Crystallography: Tl+-Substitution of Bound K+ in the E2.MGF42-.2K+ Crystal After 85 Min
Protein crystallography data
The structure of Kinetics By X-Ray Crystallography: Tl+-Substitution of Bound K+ in the E2.MGF42-.2K+ Crystal After 85 Min, PDB code: 5aw2
was solved by
H.Ogawa,
F.Cornelius,
A.Hirata,
C.Toyoshima,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 14.98 / 3.20 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 221.294, 50.625, 163.470, 90.00, 104.26, 90.00 |
| R / Rfree (%) | 28.4 / 28 |
Other elements in 5aw2:
The structure of Kinetics By X-Ray Crystallography: Tl+-Substitution of Bound K+ in the E2.MGF42-.2K+ Crystal After 85 Min also contains other interesting chemical elements:
| Magnesium | (Mg) | 2 atoms |
| Potassium | (K) | 1 atom |
| Thallium | (Tl) | 3 atoms |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Kinetics By X-Ray Crystallography: Tl+-Substitution of Bound K+ in the E2.MGF42-.2K+ Crystal After 85 Min
(pdb code 5aw2). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 4 binding sites of Fluorine where determined in the Kinetics By X-Ray Crystallography: Tl+-Substitution of Bound K+ in the E2.MGF42-.2K+ Crystal After 85 Min, PDB code: 5aw2:
Jump to Fluorine binding site number: 1; 2; 3; 4;
In total 4 binding sites of Fluorine where determined in the Kinetics By X-Ray Crystallography: Tl+-Substitution of Bound K+ in the E2.MGF42-.2K+ Crystal After 85 Min, PDB code: 5aw2:
Jump to Fluorine binding site number: 1; 2; 3; 4;
Fluorine binding site 1 out of 4 in 5aw2
Go back to
Fluorine binding site 1 out
of 4 in the Kinetics By X-Ray Crystallography: Tl+-Substitution of Bound K+ in the E2.MGF42-.2K+ Crystal After 85 Min

Mono view
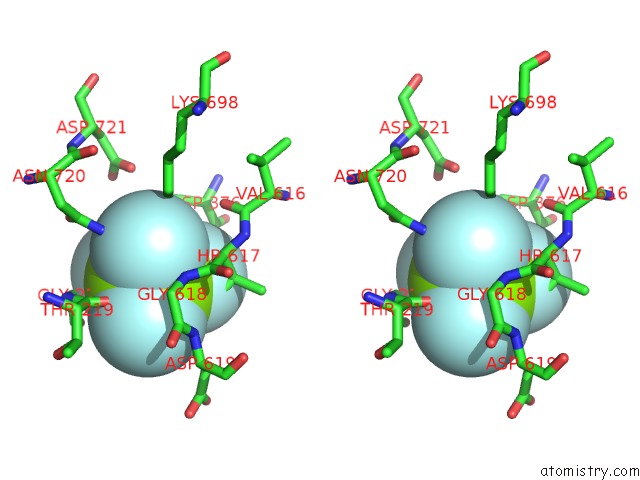
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Kinetics By X-Ray Crystallography: Tl+-Substitution of Bound K+ in the E2.MGF42-.2K+ Crystal After 85 Min within 5.0Å range:
|
Fluorine binding site 2 out of 4 in 5aw2
Go back to
Fluorine binding site 2 out
of 4 in the Kinetics By X-Ray Crystallography: Tl+-Substitution of Bound K+ in the E2.MGF42-.2K+ Crystal After 85 Min
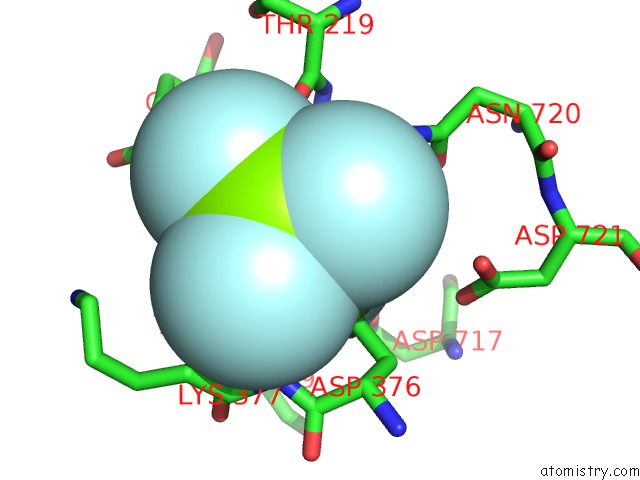
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Kinetics By X-Ray Crystallography: Tl+-Substitution of Bound K+ in the E2.MGF42-.2K+ Crystal After 85 Min within 5.0Å range:
|
Fluorine binding site 3 out of 4 in 5aw2
Go back to
Fluorine binding site 3 out
of 4 in the Kinetics By X-Ray Crystallography: Tl+-Substitution of Bound K+ in the E2.MGF42-.2K+ Crystal After 85 Min

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Kinetics By X-Ray Crystallography: Tl+-Substitution of Bound K+ in the E2.MGF42-.2K+ Crystal After 85 Min within 5.0Å range:
|
Fluorine binding site 4 out of 4 in 5aw2
Go back to
Fluorine binding site 4 out
of 4 in the Kinetics By X-Ray Crystallography: Tl+-Substitution of Bound K+ in the E2.MGF42-.2K+ Crystal After 85 Min

Mono view
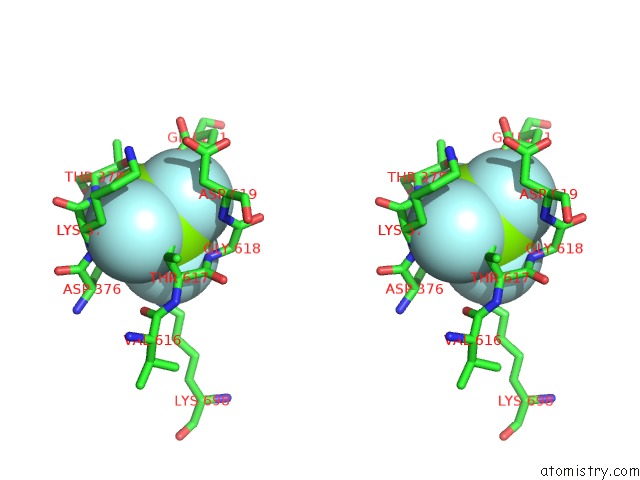
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 4 of Kinetics By X-Ray Crystallography: Tl+-Substitution of Bound K+ in the E2.MGF42-.2K+ Crystal After 85 Min within 5.0Å range:
|
Reference:
H.Ogawa,
F.Cornelius,
A.Hirata,
C.Toyoshima.
Sequential Substitution of K(+) Bound to Na(+),K(+)-Atpase Visualized By X-Ray Crystallography. Nat Commun V. 6 8004 2015.
ISSN: ESSN 2041-1723
PubMed: 26258479
DOI: 10.1038/NCOMMS9004
Page generated: Tue Jul 15 02:24:46 2025
ISSN: ESSN 2041-1723
PubMed: 26258479
DOI: 10.1038/NCOMMS9004
Last articles
F in 7JHWF in 7JHD
F in 7I18
F in 7I2F
F in 7I2M
F in 7I2A
F in 7I2D
F in 7HNS
F in 7HOG
F in 7HO4