Fluorine »
PDB 5le1-5lz5 »
5lws »
Fluorine in PDB 5lws: Endothiapepsin in Complex with Fragment 177 and A Derivative Thereof
Enzymatic activity of Endothiapepsin in Complex with Fragment 177 and A Derivative Thereof
All present enzymatic activity of Endothiapepsin in Complex with Fragment 177 and A Derivative Thereof:
3.4.23.22;
3.4.23.22;
Protein crystallography data
The structure of Endothiapepsin in Complex with Fragment 177 and A Derivative Thereof, PDB code: 5lws
was solved by
J.Schiebel,
A.Heine,
G.Klebe,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 36.55 / 1.03 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 45.327, 73.095, 53.029, 90.00, 109.83, 90.00 |
| R / Rfree (%) | 11.6 / 13.2 |
Other elements in 5lws:
The structure of Endothiapepsin in Complex with Fragment 177 and A Derivative Thereof also contains other interesting chemical elements:
| Chlorine | (Cl) | 2 atoms |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Endothiapepsin in Complex with Fragment 177 and A Derivative Thereof
(pdb code 5lws). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 6 binding sites of Fluorine where determined in the Endothiapepsin in Complex with Fragment 177 and A Derivative Thereof, PDB code: 5lws:
Jump to Fluorine binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Fluorine where determined in the Endothiapepsin in Complex with Fragment 177 and A Derivative Thereof, PDB code: 5lws:
Jump to Fluorine binding site number: 1; 2; 3; 4; 5; 6;
Fluorine binding site 1 out of 6 in 5lws
Go back to
Fluorine binding site 1 out
of 6 in the Endothiapepsin in Complex with Fragment 177 and A Derivative Thereof
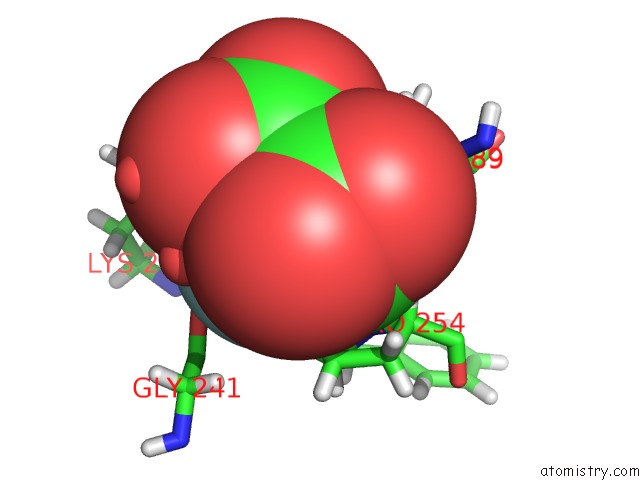
Mono view
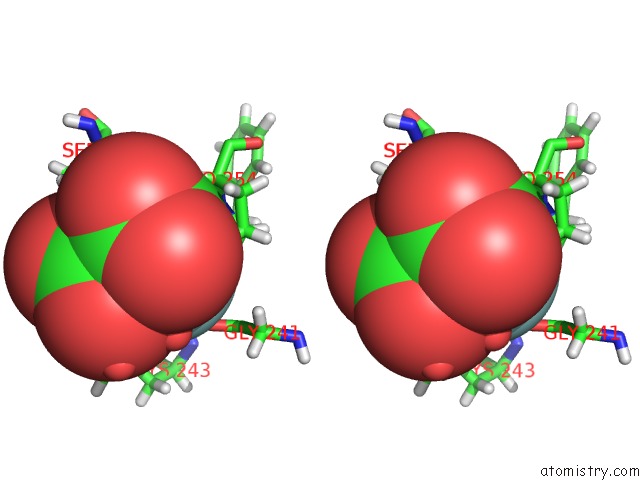
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Endothiapepsin in Complex with Fragment 177 and A Derivative Thereof within 5.0Å range:
|
Fluorine binding site 2 out of 6 in 5lws
Go back to
Fluorine binding site 2 out
of 6 in the Endothiapepsin in Complex with Fragment 177 and A Derivative Thereof
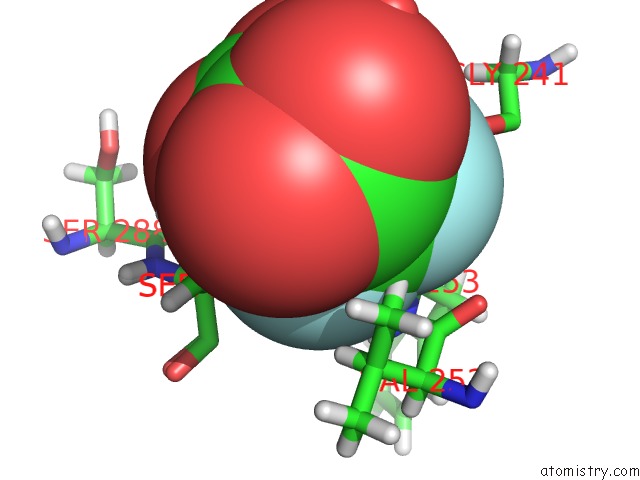
Mono view
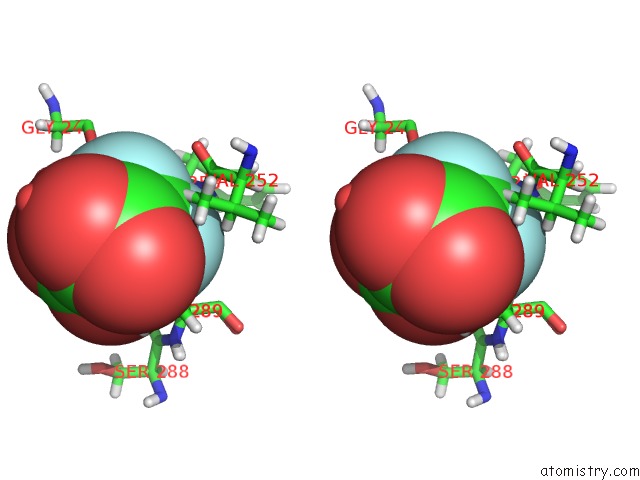
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Endothiapepsin in Complex with Fragment 177 and A Derivative Thereof within 5.0Å range:
|
Fluorine binding site 3 out of 6 in 5lws
Go back to
Fluorine binding site 3 out
of 6 in the Endothiapepsin in Complex with Fragment 177 and A Derivative Thereof
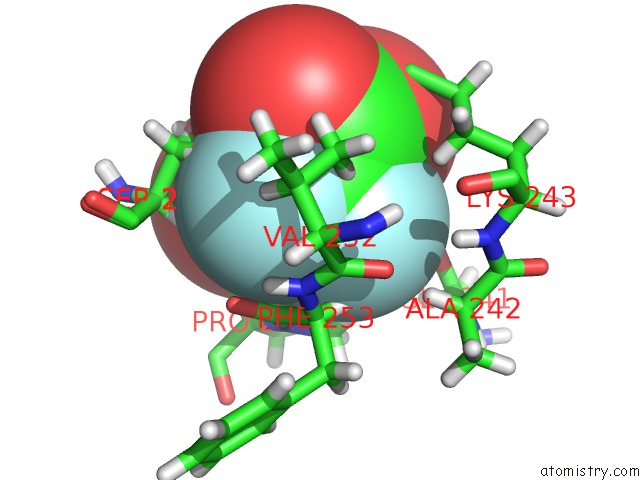
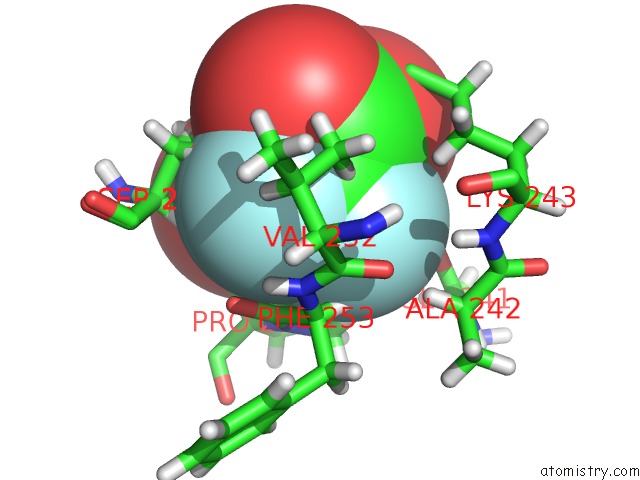
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Endothiapepsin in Complex with Fragment 177 and A Derivative Thereof within 5.0Å range:
|
Fluorine binding site 4 out of 6 in 5lws
Go back to
Fluorine binding site 4 out
of 6 in the Endothiapepsin in Complex with Fragment 177 and A Derivative Thereof
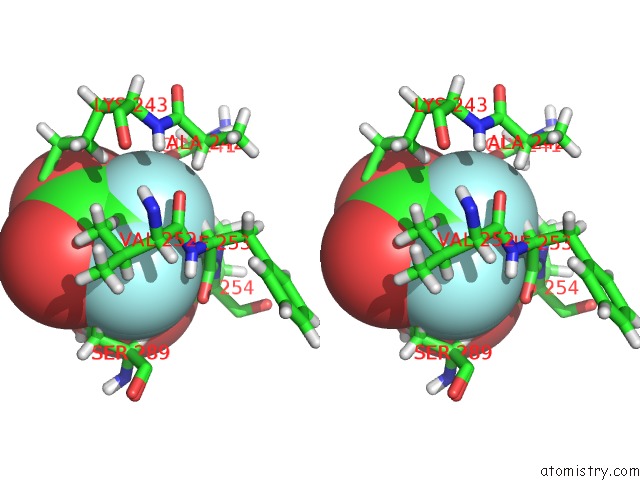
Mono view
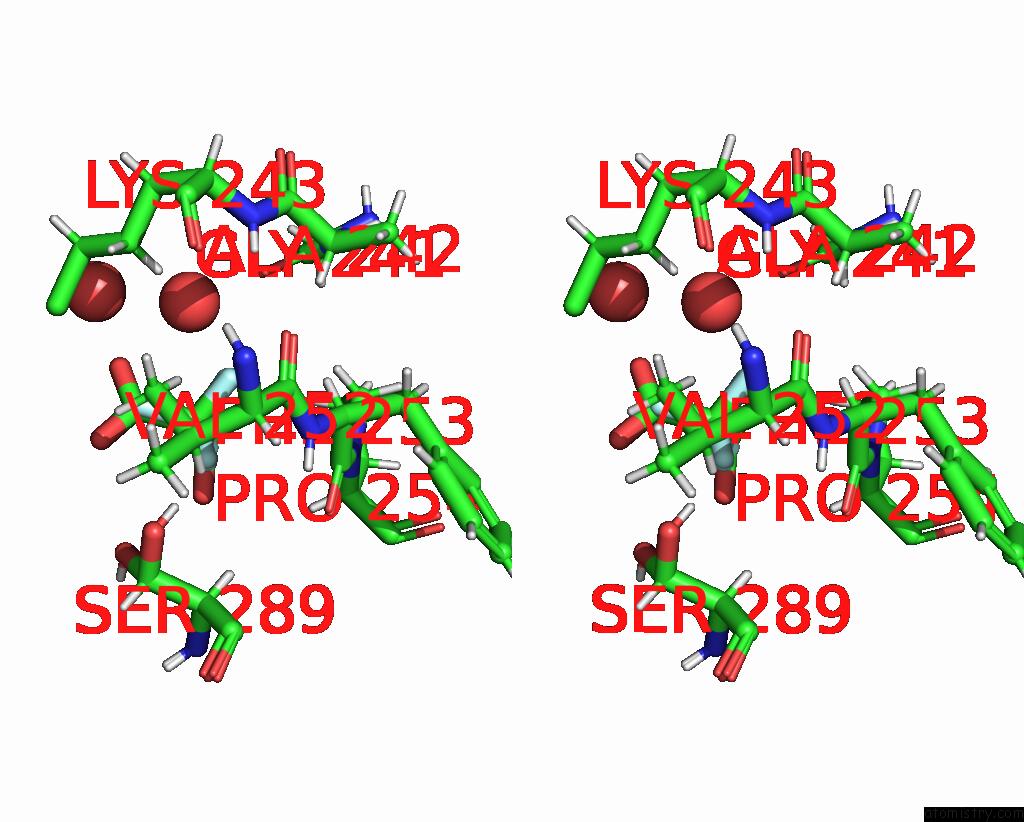
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 4 of Endothiapepsin in Complex with Fragment 177 and A Derivative Thereof within 5.0Å range:
|
Fluorine binding site 5 out of 6 in 5lws
Go back to
Fluorine binding site 5 out
of 6 in the Endothiapepsin in Complex with Fragment 177 and A Derivative Thereof
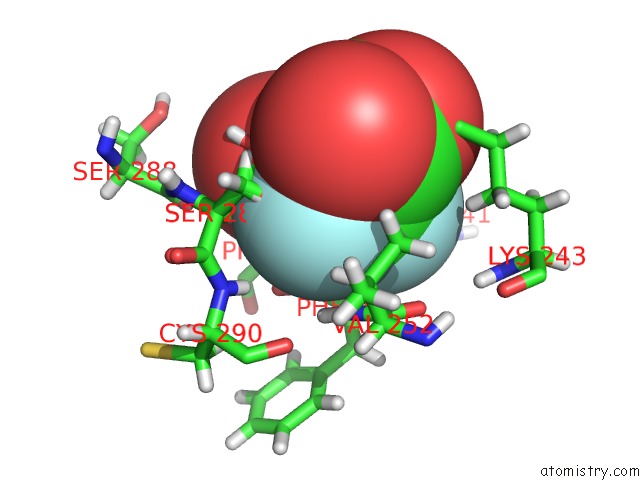
Mono view
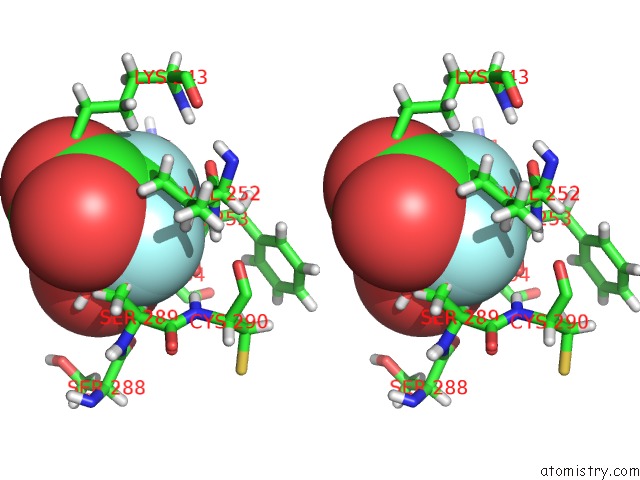
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 5 of Endothiapepsin in Complex with Fragment 177 and A Derivative Thereof within 5.0Å range:
|
Fluorine binding site 6 out of 6 in 5lws
Go back to
Fluorine binding site 6 out
of 6 in the Endothiapepsin in Complex with Fragment 177 and A Derivative Thereof
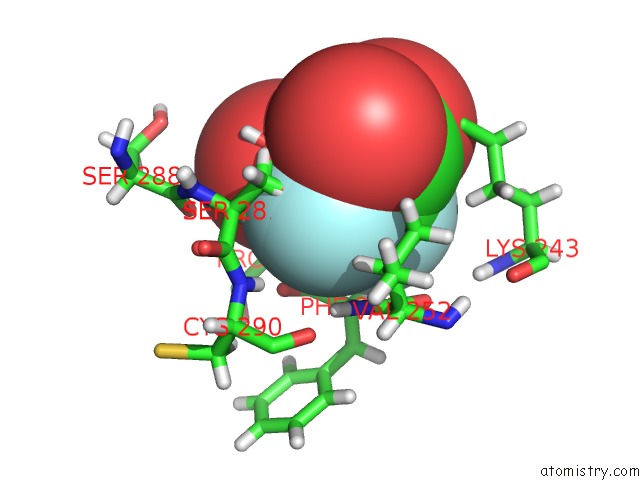
Mono view
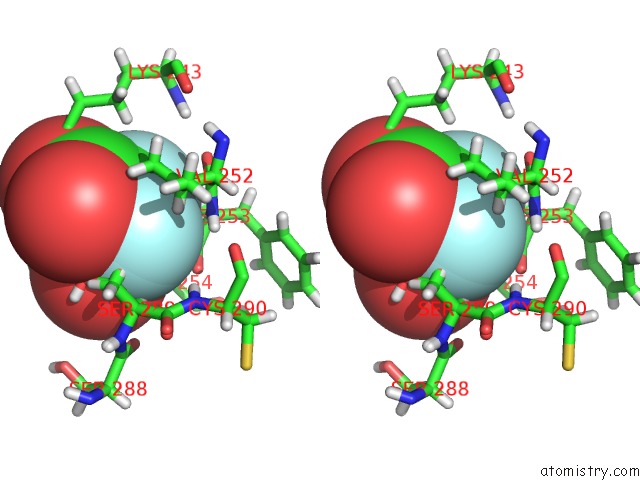
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 6 of Endothiapepsin in Complex with Fragment 177 and A Derivative Thereof within 5.0Å range:
|
Reference:
J.Cramer,
J.Schiebel,
T.Wulsdorf,
K.Grohe,
E.E.Najbauer,
F.R.Ehrmann,
N.Radeva,
N.Zitzer,
U.Linne,
R.Linser,
A.Heine,
G.Klebe.
A False-Positive Screening Hit in Fragment-Based Lead Discovery: Watch Out For the Red Herring. Angew. Chem. Int. Ed. Engl. V. 56 1908 2017.
ISSN: ESSN 1521-3773
PubMed: 28097765
DOI: 10.1002/ANIE.201609824
Page generated: Tue Jul 15 05:06:48 2025
ISSN: ESSN 1521-3773
PubMed: 28097765
DOI: 10.1002/ANIE.201609824
Last articles
F in 6BY8F in 6BXH
F in 6BXY
F in 6BX5
F in 6BX4
F in 6BU6
F in 6BU3
F in 6BT6
F in 6BVN
F in 6BVF