Fluorine »
PDB 6g0w-6gqm »
6gfz »
Fluorine in PDB 6gfz: Pvhl:Elob:Eloc in Complex with Modified VH032 Containing (3S,4S)-3- Fluoro-4-Hydroxyproline (Ligand 14B)
Protein crystallography data
The structure of Pvhl:Elob:Eloc in Complex with Modified VH032 Containing (3S,4S)-3- Fluoro-4-Hydroxyproline (Ligand 14B), PDB code: 6gfz
was solved by
M.S.Gadd,
A.Testa,
A.Ciulli,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 49.01 / 2.30 |
| Space group | P 41 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 93.609, 93.609, 364.511, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 19.5 / 24.5 |
Other elements in 6gfz:
The structure of Pvhl:Elob:Eloc in Complex with Modified VH032 Containing (3S,4S)-3- Fluoro-4-Hydroxyproline (Ligand 14B) also contains other interesting chemical elements:
| Arsenic | (As) | 12 atoms |
Fluorine Binding Sites:
The binding sites of Fluorine atom in the Pvhl:Elob:Eloc in Complex with Modified VH032 Containing (3S,4S)-3- Fluoro-4-Hydroxyproline (Ligand 14B)
(pdb code 6gfz). This binding sites where shown within
5.0 Angstroms radius around Fluorine atom.
In total 4 binding sites of Fluorine where determined in the Pvhl:Elob:Eloc in Complex with Modified VH032 Containing (3S,4S)-3- Fluoro-4-Hydroxyproline (Ligand 14B), PDB code: 6gfz:
Jump to Fluorine binding site number: 1; 2; 3; 4;
In total 4 binding sites of Fluorine where determined in the Pvhl:Elob:Eloc in Complex with Modified VH032 Containing (3S,4S)-3- Fluoro-4-Hydroxyproline (Ligand 14B), PDB code: 6gfz:
Jump to Fluorine binding site number: 1; 2; 3; 4;
Fluorine binding site 1 out of 4 in 6gfz
Go back to
Fluorine binding site 1 out
of 4 in the Pvhl:Elob:Eloc in Complex with Modified VH032 Containing (3S,4S)-3- Fluoro-4-Hydroxyproline (Ligand 14B)
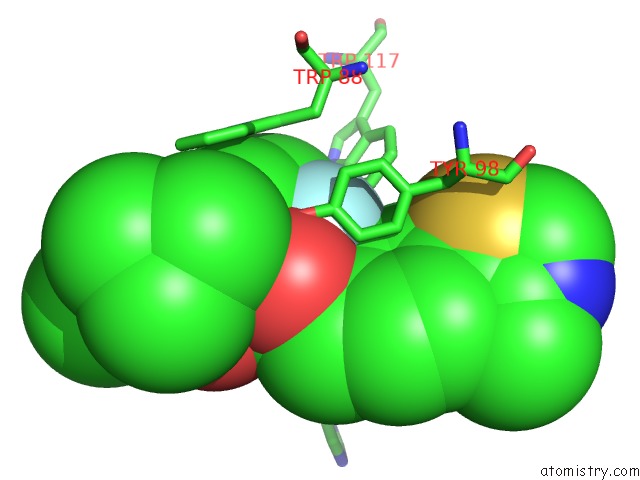
Mono view
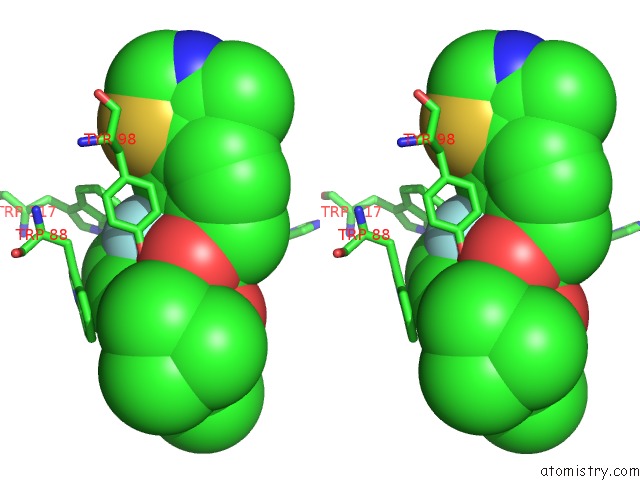
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 1 of Pvhl:Elob:Eloc in Complex with Modified VH032 Containing (3S,4S)-3- Fluoro-4-Hydroxyproline (Ligand 14B) within 5.0Å range:
|
Fluorine binding site 2 out of 4 in 6gfz
Go back to
Fluorine binding site 2 out
of 4 in the Pvhl:Elob:Eloc in Complex with Modified VH032 Containing (3S,4S)-3- Fluoro-4-Hydroxyproline (Ligand 14B)
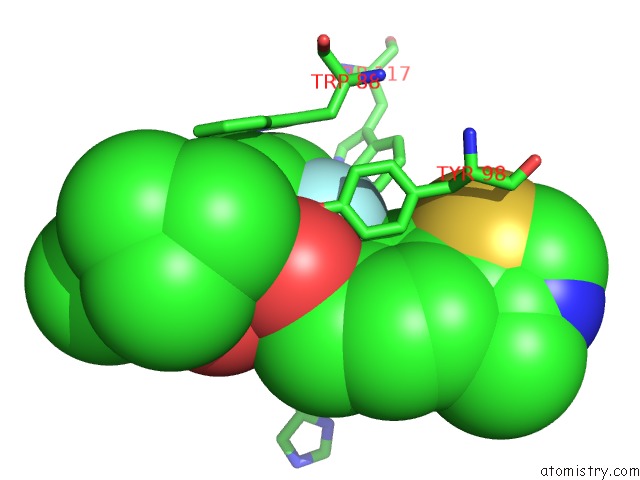
Mono view
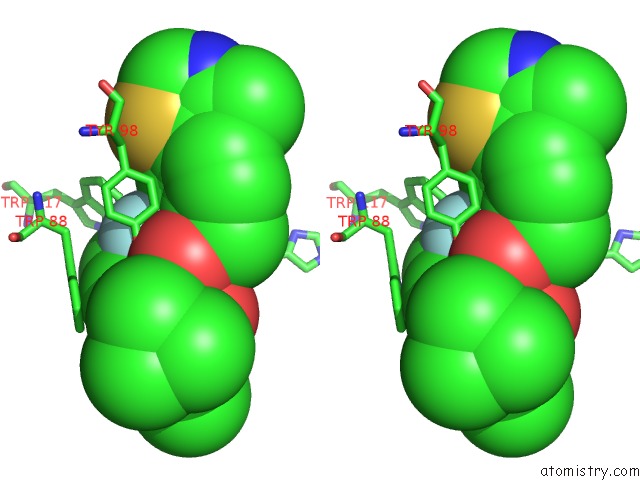
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 2 of Pvhl:Elob:Eloc in Complex with Modified VH032 Containing (3S,4S)-3- Fluoro-4-Hydroxyproline (Ligand 14B) within 5.0Å range:
|
Fluorine binding site 3 out of 4 in 6gfz
Go back to
Fluorine binding site 3 out
of 4 in the Pvhl:Elob:Eloc in Complex with Modified VH032 Containing (3S,4S)-3- Fluoro-4-Hydroxyproline (Ligand 14B)
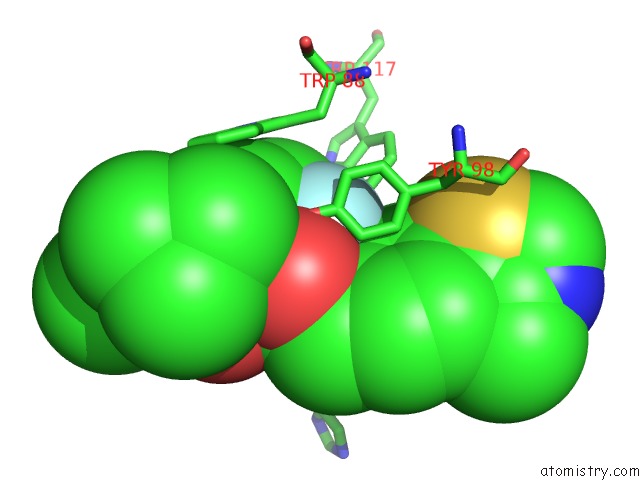
Mono view
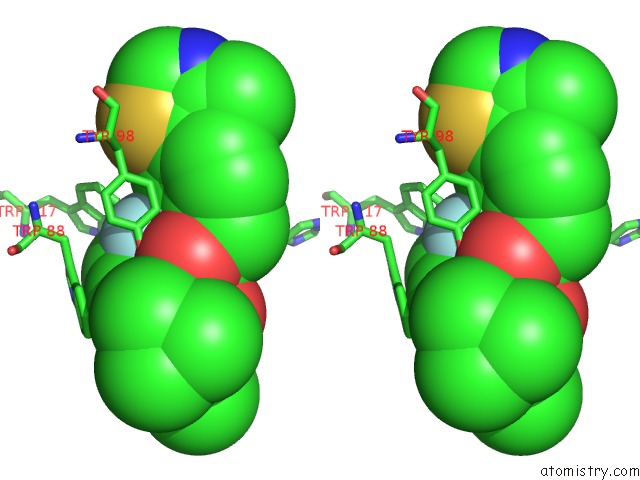
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 3 of Pvhl:Elob:Eloc in Complex with Modified VH032 Containing (3S,4S)-3- Fluoro-4-Hydroxyproline (Ligand 14B) within 5.0Å range:
|
Fluorine binding site 4 out of 4 in 6gfz
Go back to
Fluorine binding site 4 out
of 4 in the Pvhl:Elob:Eloc in Complex with Modified VH032 Containing (3S,4S)-3- Fluoro-4-Hydroxyproline (Ligand 14B)
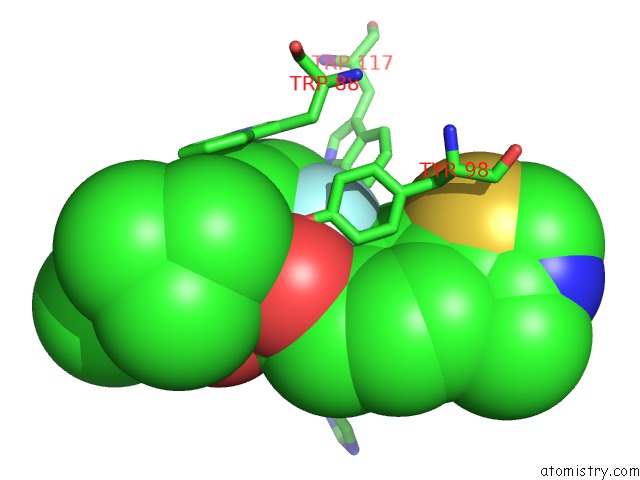
Mono view
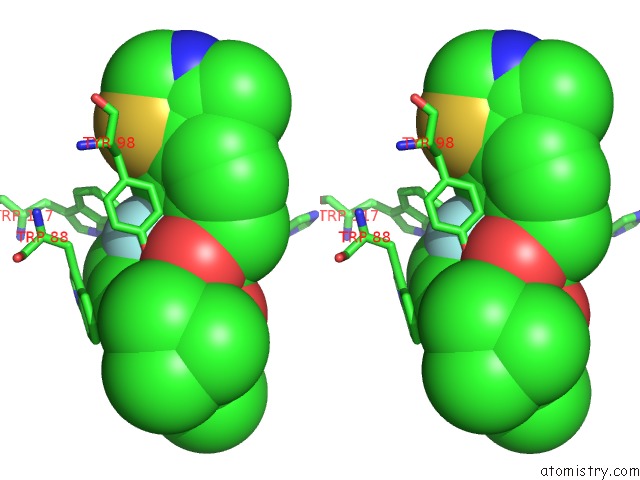
Stereo pair view

Mono view

Stereo pair view
A full contact list of Fluorine with other atoms in the F binding
site number 4 of Pvhl:Elob:Eloc in Complex with Modified VH032 Containing (3S,4S)-3- Fluoro-4-Hydroxyproline (Ligand 14B) within 5.0Å range:
|
Reference:
A.Testa,
X.Lucas,
G.V.Castro,
K.H.Chan,
J.E.Wright,
A.C.Runcie,
M.S.Gadd,
W.T.A.Harrison,
E.J.Ko,
D.Fletcher,
A.Ciulli.
3-Fluoro-4-Hydroxyprolines: Synthesis, Conformational Analysis, and Stereoselective Recognition By the Vhl E3 Ubiquitin Ligase For Targeted Protein Degradation. J. Am. Chem. Soc. V. 140 9299 2018.
ISSN: ESSN 1520-5126
PubMed: 29949369
DOI: 10.1021/JACS.8B05807
Page generated: Tue Jul 15 11:52:55 2025
ISSN: ESSN 1520-5126
PubMed: 29949369
DOI: 10.1021/JACS.8B05807
Last articles
F in 7M0UF in 7M0N
F in 7M0T
F in 7M00
F in 7M01
F in 7M03
F in 7M02
F in 7LZU
F in 7LZW
F in 7LY8